Organic Chemists from Industry and academics to Interact on Spectroscopy Techniques for Organic Compounds ie NMR, MASS, IR, UV Etc. Starters, Learners, advanced, all alike, contains content which is basic or advanced, by Dr Anthony Melvin Crasto, Worlddrugtracker, email me ........... amcrasto@gmail.com, call +91 9323115463 India skype amcrasto64
Pages
▼
Pages
▼
Saturday, 24 February 2018
Thursday, 15 February 2018
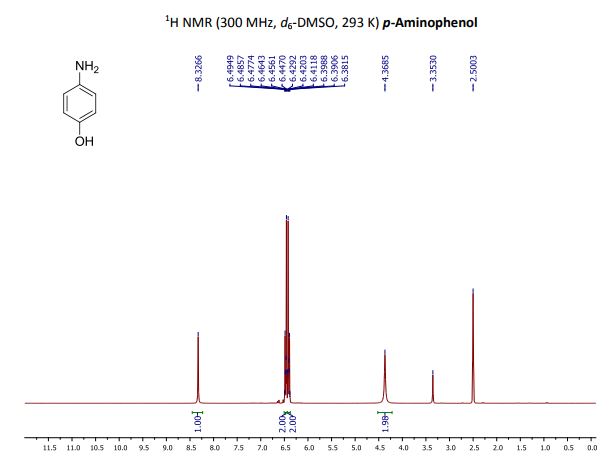
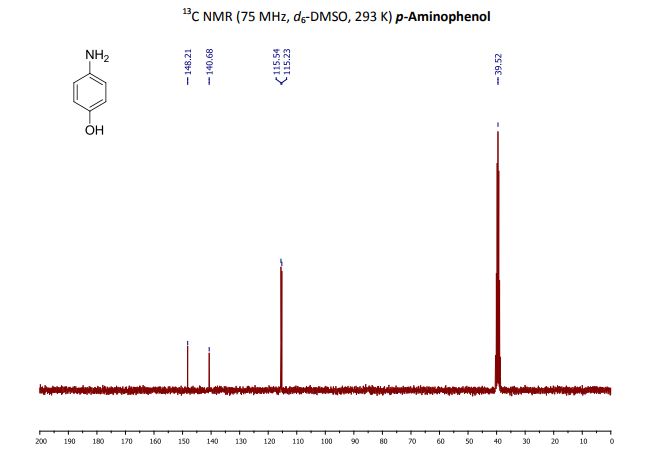
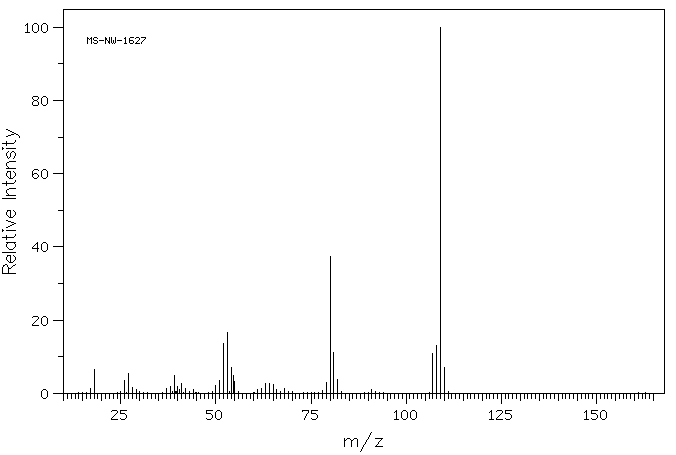
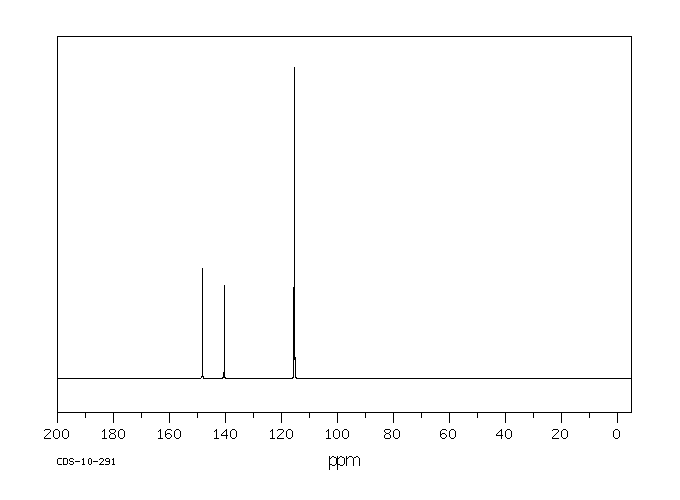
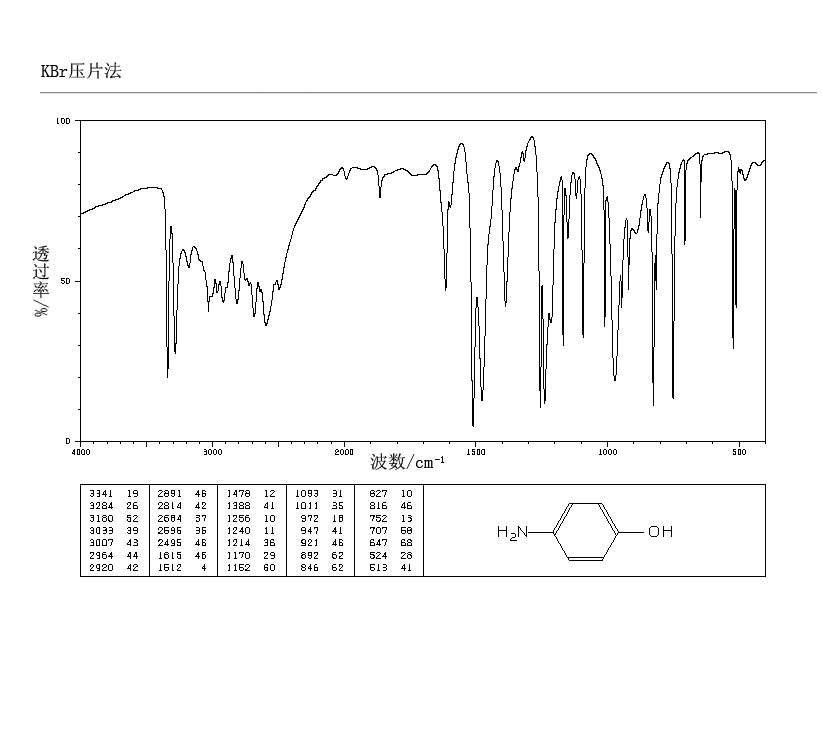
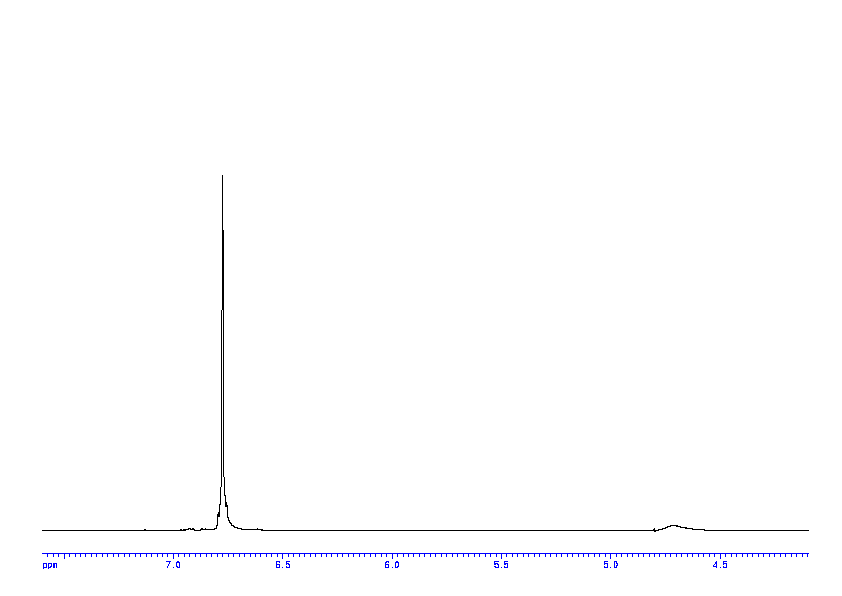
p-Aminophenol
p-Aminophenol [123-30-8].
M.p. 182 °C; 1H NMR (300 MHz, d6-DMSO): 4.37 (br s, 2H, NH2), 6.37-6.44 (m, 2HAr), 6.44-6.50 (m, 2HAr), 8.33 (br s, 1H, OH);
13C NMR (75 MHz, d6-DMSO): δ 115.2 (2 CHAr), 115.5 (2 CHAr), 140.7 (Cq Ar), 148.2 (Cq Ar);
IR (ATR) max: 3338, 3279, 1471; MS (ESI+ ): 110.1 ([M+H]+ , 100).
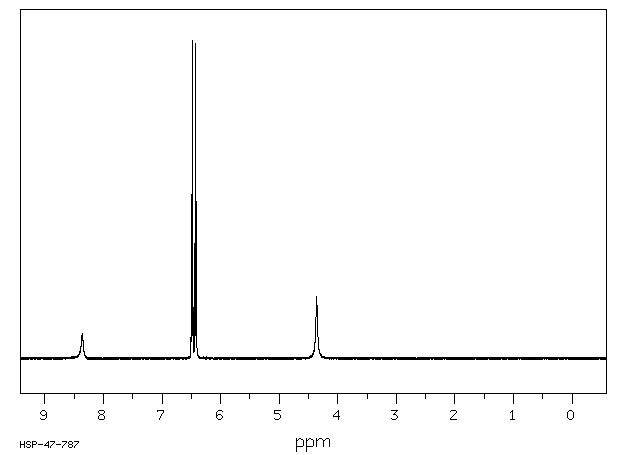
1D 1H ABOVE
![2D [1H,1H]-TOCSY, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/HH_TOCSY.png)
2D [1H,1H]-TOCSY ABOVE
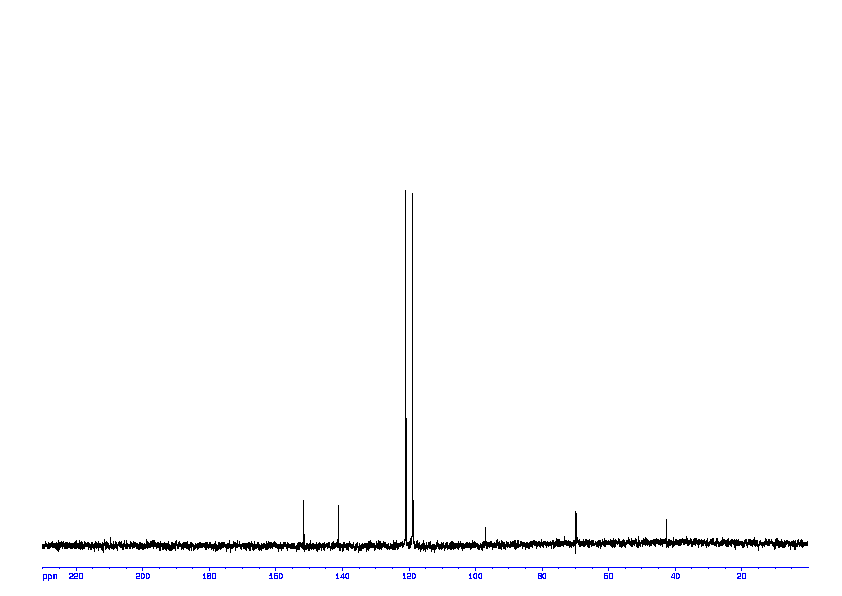
1D 13C ABOVE
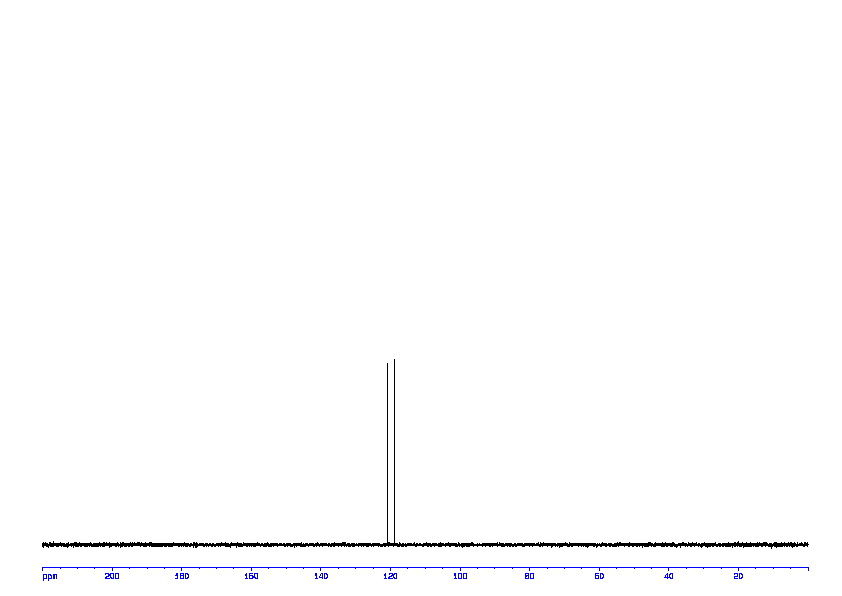
1D DEPT90 ABOVE
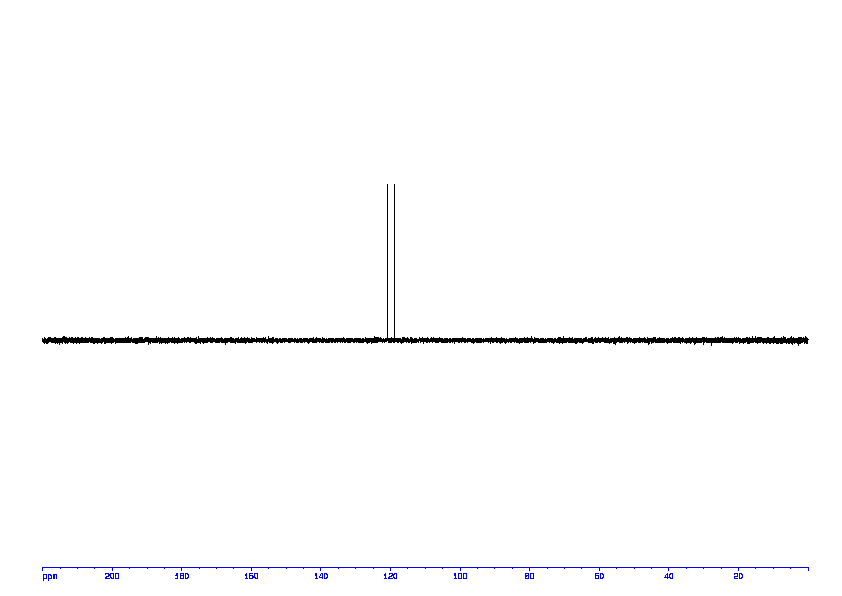
1D DEPT135 ABOVE
![2D [1H,13C]-HSQC, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/1H_13C_HSQC.png)
2D [1H,13C]-HSQC ABOVE
![2D [1H,13C]-HMBC, 7.4 spectrum for 4-Aminophenol](http://www.bmrb.wisc.edu/ftp/pub/bmrb/metabolomics/entry_directories/bmse000462/nmr/set01/spectra/1H_13C_HMBC.png)
2D [1H,13C]-HMBC ABOVE
Thursday, 8 February 2018
A sustainable procedure toward alkyl arylacetates: palladium-catalysed direct carbonylation of benzyl alcohols in organic carbonates
Green Chem., 2018, Advance Article
DOI: 10.1039/C7GC03619A, Communication
DOI: 10.1039/C7GC03619A, Communication
Yahui Li, Zechao Wang, Xiao-Feng Wu
A sustainable procedure for the synthesis of various alkyl arylacetates from benzyl alcohols has been developed
A sustainable procedure for the synthesis of various alkyl arylacetates from benzyl alcohols has been developed
A sustainable procedure toward alkyl arylacetates: palladium-catalysed direct carbonylation of benzyl alcohols in organic carbonates
Author affiliations
* Corresponding authors
a Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
b Department of Chemistry, Zhejiang Sci-Tech University, Xiasha Campus, Hangzhou 310018, People's Republic of China
E-mail: Xiao-Feng.Wu@catalysis.de
E-mail: Xiao-Feng.Wu@catalysis.de
Abstract
A sustainable procedure for the synthesis of various alkyl arylacetates from benzyl alcohols has been developed. With palladium as the catalyst and organic carbonates as the green solvent and in situ activator, benzyl alcohols were carbonylated in an efficient manner without any halogen additives.
Ethyl 2-phenylacetate1H NMR (300 MHz, Chloroform-d) δ 7.32 – 7.08 (m, 5H), 4.08 (q, J = 7.1 Hz, 2H), 3.54 (s, 2H), 1.18 (t, J = 7.1 Hz, 3H).
13C NMR (75 MHz, CDCl3) δ 171.61, 134.17, 129.24, 128.54, 127.03, 60.85, 41.45, 14.18.
Unconventional Method for the Synthesis of 3-Carboxyethyl-4-formyl(hydroxy)-5-arylpyrazoles
Unconventional Method for Synthesis of 3-Carboxyethyl-4-formyl(hydroxy)-5-aryl-N-arylpyrazoles
Michael J. V. da Silva†, Julia Poletto†, Andrey P. Jacomini†, Karlos E. Pianoski†, Davana S. Gonçalves†, Gessica M. Ribeiro†, Samara M. de S. Melo†, Davi F. Back‡, Sidnei Moura§, and Fernanda A. Rosa*† 

† Departamento de Química, Universidade Estadual de Maringá (UEM), 87030-900 Maringá, PR, Brazil
‡ Departamento de Química, Universidade Federal de Santa Maria (UFSM), 97110-970 Santa Maria, RS, Brazil
§ Instituto de Biotecnologia, Universidade de Caxias do Sul (UCS), 295070-560 Caxias do Sul, RS, Brazil
J. Org. Chem., 2017, 82 (23), pp 12590–12602
DOI: 10.1021/acs.joc.7b02361
Publication Date (Web): November 2, 2017
*E-mail: farosa@uem.br
Abstract
An alternative highly regioselective synthetic method for the preparation of 3,5-disubstituted 4-formyl-N-arylpyrazoles in a one-pot procedure is reported. The methodology developed was based on the regiochemical control of the cyclocondensation reaction of β-enamino diketones with arylhydrazines.
Structural modifications in the β-enamino diketone system allied to the Lewis acid carbonyl activator BF3 were strategically employed for this control. Also a one-pot method for the preparation of 3,5-disubstituted 4-hydroxymethyl-N-arylpyrazole derivatives from the β-enamino diketone and arylhydrazine substrates is described.

4-Formyl-N-arylpyrazole substrates occupy a prominent position in the field of organic synthesis since they are key intermediates in obtaining a wide range of biologically active compounds. Because of the synthetic versatility of the 4-formyl-N-arylpyrazole skeleton, their synthesis has been extensively explored. In an extension of their previously published research,
Rosa and co-workers at Universidade Estadual de Maringá described a one-pot synthetic method that regioselectively produced 3,5-disubstituted-4-formyl-N-arylpyrazoles . The β-enamino diketone starting materials were readily synthesized via published procedures. High regioselectivity was secured via the use of BF3·OEt2 as the carbonyl activator and a bulky amine as the enamine component. Acetonitrile proved to be the most suitable solvent for the reaction.
After an aqueous workup, the desired pyrazoles were obtained in excellent yields. A variety of functional groups were tolerated on the two aryl substituents. This operationally simple procedure afforded the 4-formyl-N-arylpyrazoles in high yields, regioselectively. Furthermore, the formyl group could be reduced in situ with sodium borohydride to generate the corresponding 4-hydroxymethyl-N-arylpyrazoles.
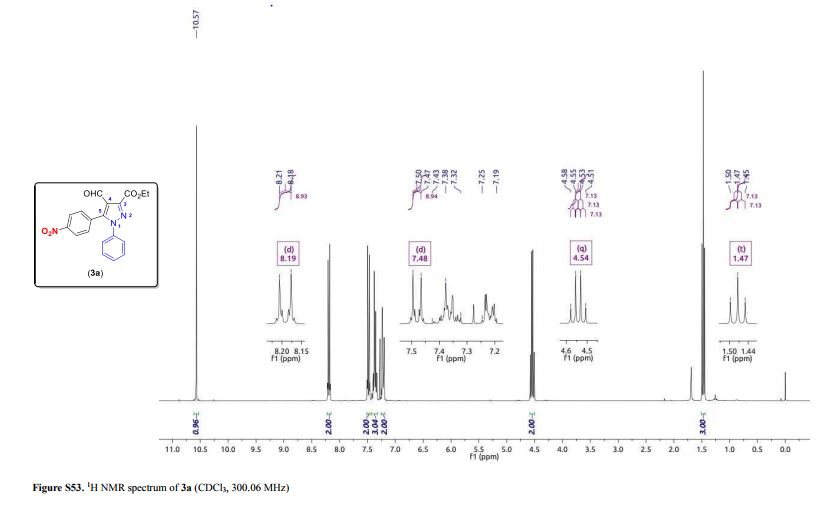
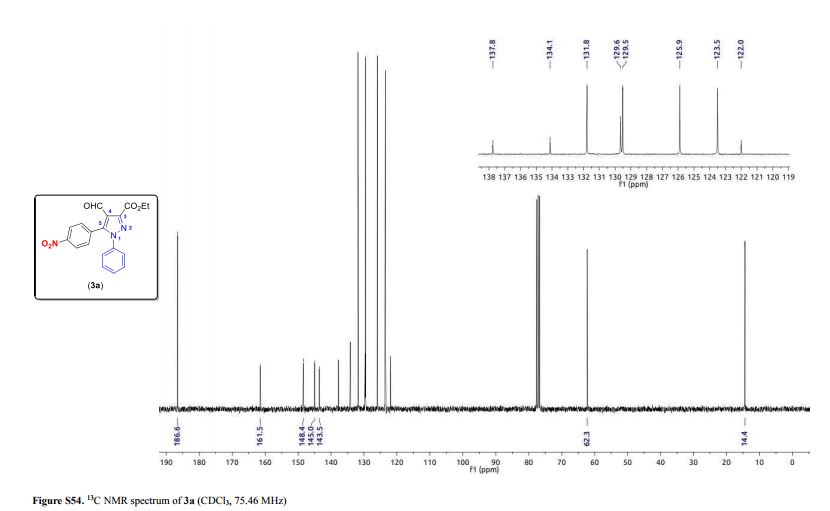
3-(Ethoxycarbonyl)-4-formyl-5-(4-nitrophenyl)-1-phenyl-1H-pyrazole (3a)
Light yellow solid; yield: 0.150 g (82%); mp 147.0–149.2 °C;
1H NMR (300.06 MHz, CDCl3) δ (ppm) 1.47 (t, 3H, J = 7.1 Hz, O–CH2–CH3), 4.54 (q, 2H, J = 7.1 Hz, O–CH2-CH3), 7.19–7.25 (m, 2H, Ph), 7.32–7.43 (m, 3H, Ph), 7.48 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 8.19 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 10.57 (s, 1H, CHO);
13C NMR (75.46 MHz, CDCl3) δ (ppm) 14.4 (O–CH2–CH3), 62.3 (O-CH2–CH3), 122.0 (C4), 123.5 (4-NO2C6H4), 125.9 (Ph), 129.5 (Ph), 129.6 (Ph), 131.8 (4-NO2C6H4), 134.1 (4-NO2C6H4), 137.8 (Ph), 143.5 (C5), 145.0 (C3), 148.4 (4-NO2C6H4), 161.5 (COOEt), 186.6 (CHO);
HRMS (ESI+): calcd for C19H16N3O5+, [M+H]+: 366.1084, found 366.1101.
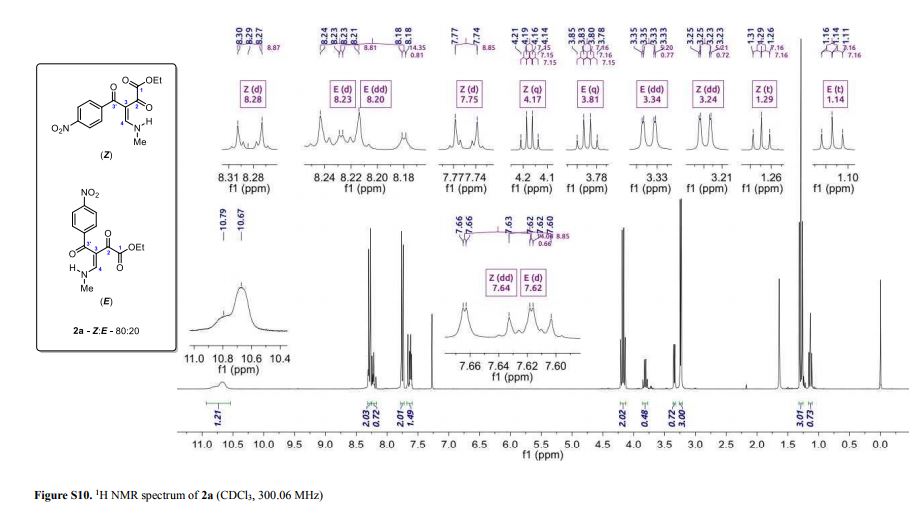
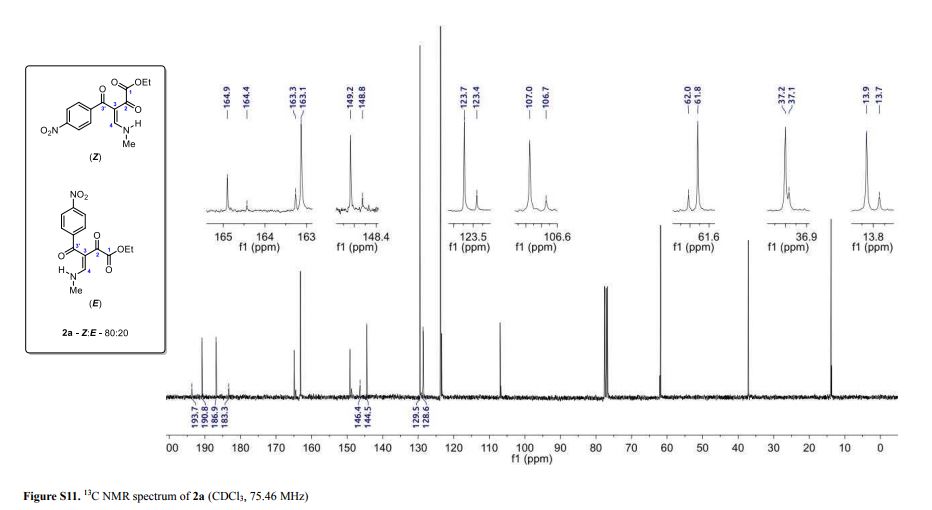
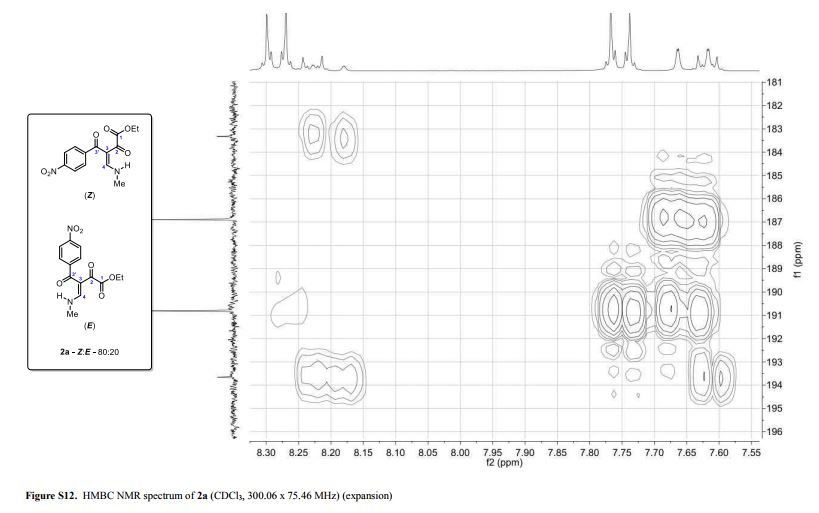
(Z and E)-4-(Methylamino)-3-(4-nitrobenzoyl)-2-oxobut-3-enoic Acid Ethyl Ester
(Z and E)-4-(Methylamino)-3-(4-nitrobenzoyl)-2-oxobut-3-enoic Acid Ethyl Ester (2a)
Light yellow solid; yield: 0.276 g (90%); Z/E ratio in CDCl3: 80/20; mp 143.8–145.3 °C;
1H NMR (300.06 MHz, CDCl3) δ (ppm) (Z) 1.29 (t, 3H, J = 7.2 Hz, O–CH2–CH3), 3.24 (dd, 3H, J = 5.2, 0.7 Hz, NH-CH3), 4.17 (q, 2H, J = 7.2 Hz, O–CH2-CH3), 7.64 (dd, 1H, J = 14.1, 0.7 Hz, H4), 7.75 (d, 2H, J = 8.8 Hz, 4-NO2C6H4), 8.28 (d, 2H, J = 8.9 Hz, 4-NO2C6H4), 10.67 (bs, 1H, NH); (E) 1.14 (t, 3H, J = 7.2 Hz, O–CH2–CH3), 3.34 (dd, 3H, J = 5.2, 0.8 Hz, NH-CH3), 3.81 (q, 2H, J = 7.2 Hz, O–CH2-CH3), 7.62 (d, 2H, J = 8.8 Hz, 4-NO2C6H4), 8.20 (dd, 1H, J = 14.3, 0.8 Hz, H4), 8.23 (d, 2H, J= 8.8 Hz, 4-NO2C6H4), 10.79 (bs, 1H, NH);
13C NMR (75.46 MHz, CDCl3) δ (ppm) (Z) 13.9 (O–CH2–CH3), 37.2 (NH-CH3), 61.8 (O-CH2–CH3), 107.0 (C3), 123.7, 129.5, 144.5, 149.2 (4-NO2C6H4), 163.1 (C4), 164.9 (COOEt), 186.9 (C2), 190.8 (C3′); (E) 13.7 (O–CH2–CH3), 37.1 (NH-CH3), 62.0 (O-CH2–CH3), 106.7 (C3), 123.4, 128.6, 146.4, 148.8 (4-NO2C6H4), 163.3 (C4), 164.4 (COOEt), 183.3 (C2), 193.7 (C3′);
HRMS (ESI+): calcd for C14H15N2O6+, [M+H]+: 307.0925, found 307.0938.
J. Org. Chem., 2017, 82 (23), pp 12590–12602
DOI: 10.1021/acs.joc.7b02361