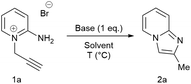
Rapid, metal-free and aqueous synthesis of imidazo[1,2-a]pyridine under ambient conditions
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC01601D, Communication
DOI: 10.1039/C6GC01601D, Communication
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Michael R. Chapman, Maria H. T. Kwan, Georgina E. King, Benjamin A. Kyffin, A. John Blacker, Charlotte E. Willans, Bao N. Nguyen
A route to access the privileged imidazo[1,2-a]pyridine scaffold in one step, 1-10 minutes using only aqueous NaOH, is reported.
A route to access the privileged imidazo[1,2-a]pyridine scaffold in one step, 1-10 minutes using only aqueous NaOH, is reported.
Rapid, metal-free and aqueous synthesis of imidazo[1,2-a]pyridine under ambient conditions
*Corresponding authors
aInstitute of Process Research and Development, School of Chemistry, University of Leeds, Leeds, UK
E-mail: b.nguyen@leeds.ac.uk
E-mail: b.nguyen@leeds.ac.uk
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC01601D
A novel, rapid and efficient route to imidazo[1,2-a]pyridines under ambient, aqueous and metal-free conditions is reported. The NaOH-promoted cycloisomerisations of N-propargylpyridiniums give quantitative yield in a few minutes (10 g scale). A comparison of common green metrics to current routes showed clear improvements, with at least a one order of magnitude increase in space-time-yield.
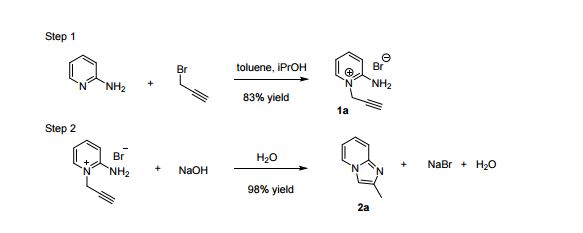
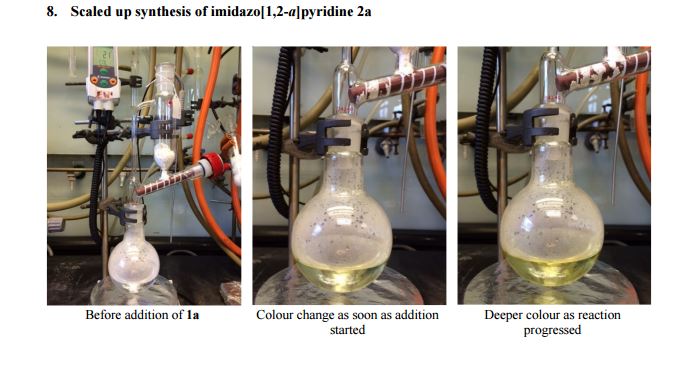
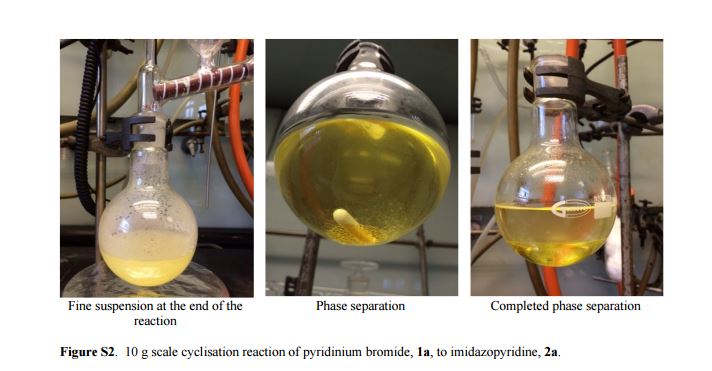
| Fig. 1 A scaled up reaction setup. (a) before reaction; (b) during addition of 1a (zoomed in); (c) phase separation at the end of the reaction (zoomed in). |
2-Aminopyridine (6.12 g, 65.0 mmol), propargyl bromide (11.6 g of an 80 wt.% solution in toluene, 78 mmol, 1.2 equiv) and 2-propanol (200 mL) charged to a round bottomed flask and stirred at 50 C for 2 hours. After which, a pale yellow solid precipitated from solution. This was filtered and washed with diethyl ether (2 x 30 mL) followed by drying in vacuo to give product 1a in 11.1 g (52 mmol, 80% isolated yield). To a stirring solution of NaOH (1.90 g, 47.5 mmol) in deionised H2O (70 mL) was added 2-amino-1- (2-propynyl)pyridinium bromide 1a (10.0 g, 47.0 mmol) via powder addition funnel (a) over a period of 5 minutes. Immediately upon addition, the solution phase turned yellow (b – d) and a yellow oil became dispersed as a distinct separate phase (e). The oil (product) was subsequently extracted into EtOAc (2 × 30 mL) (f), dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to afford imidazo[1,2-a]pyridine 2a as a spectroscopically pure pale yellow oil. Yield: 6.12 g, 98% yield.
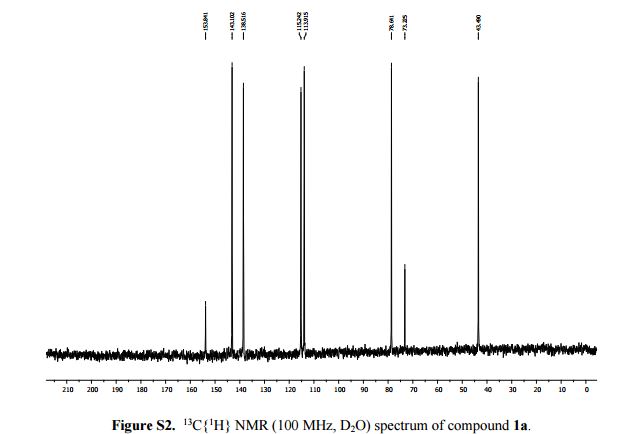
2-Amino-1-(2-propynyl)pyridinium bromide 1a: 1
1 M. Bakherad, H. N. –Isfahani, A. Keivanloo, N. Doostmohammadi, Tetrahedron Lett. 2008, 49, 3819-3822
2-Aminopyridine was reacted according to the general procedure (vide supra), affording the product as a colourless solid. Yield: 0.88 g, 83% yield.
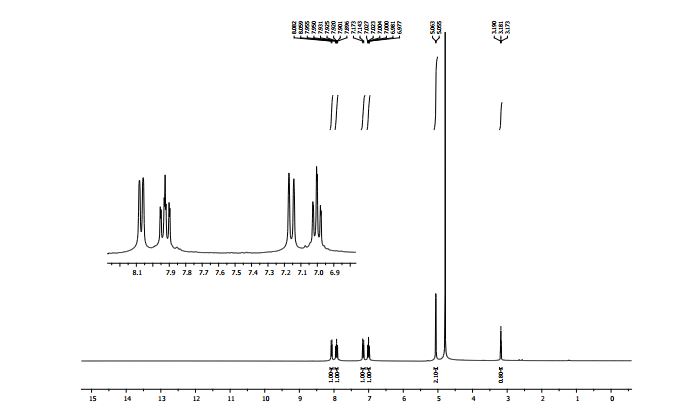
1H NMR (300 MHz, D2O): δ (ppm) 8.08 (d, J = 6.9 Hz, 1H, pyH), 7.93 (t, J = 16.2, 8.4 Hz, 1H, pyH), 7.17 (d, J = 8.4 Hz, 1H, pyH), 7.01 (t, J = 14.1, 6.9 Hz, 1H, pyH), 5.06 (d, J = 2.7 Hz, 2H, CH2), 3.18 (t, J = 5.1, 2.7 Hz, 1H, C≡CH).
13C{1H} NMR (100 MHz, D2O): δ (ppm) 153.8, 143.1, 138.5, 115.2, 113.9, 78.6, 73.2, 43.5.
HR-MS (ESI+ ): m/z 133.0756 [C8H9N2] + , calcd. [M – Br]+ 133.0760.
Anal. calcd. (%) for C8H9N2Br: C 45.10, H 4.26, N 13.15; found C 45.40, H 4.30, N 13.20.
Lit. data:1 1H NMR (500 MHz, DMSO-d6) 8.72 (s, 2H, NH2), 8.23 – 6.85 (m, 4H, pyH), 5.12 (s, 2H, CH2), 3.85 (s, 1H, CH).
13C NMR (125 MHz, DMSO-d6) 154.5, 143.6, 139.8, 115.8, 114.0, 80.5, 76.0, 43.9.
1H NMR BELOW 1a
1 M. Bakherad, H. N. –Isfahani, A. Keivanloo, N. Doostmohammadi, Tetrahedron Lett. 2008, 49, 3819-3822
2-Aminopyridine was reacted according to the general procedure (vide supra), affording the product as a colourless solid. Yield: 0.88 g, 83% yield.
1H NMR (300 MHz, D2O): δ (ppm) 8.08 (d, J = 6.9 Hz, 1H, pyH), 7.93 (t, J = 16.2, 8.4 Hz, 1H, pyH), 7.17 (d, J = 8.4 Hz, 1H, pyH), 7.01 (t, J = 14.1, 6.9 Hz, 1H, pyH), 5.06 (d, J = 2.7 Hz, 2H, CH2), 3.18 (t, J = 5.1, 2.7 Hz, 1H, C≡CH).
13C{1H} NMR (100 MHz, D2O): δ (ppm) 153.8, 143.1, 138.5, 115.2, 113.9, 78.6, 73.2, 43.5.
HR-MS (ESI+ ): m/z 133.0756 [C8H9N2] + , calcd. [M – Br]+ 133.0760.
Anal. calcd. (%) for C8H9N2Br: C 45.10, H 4.26, N 13.15; found C 45.40, H 4.30, N 13.20.
Lit. data:1 1H NMR (500 MHz, DMSO-d6) 8.72 (s, 2H, NH2), 8.23 – 6.85 (m, 4H, pyH), 5.12 (s, 2H, CH2), 3.85 (s, 1H, CH).
13C NMR (125 MHz, DMSO-d6) 154.5, 143.6, 139.8, 115.8, 114.0, 80.5, 76.0, 43.9.
1H NMR BELOW 1a
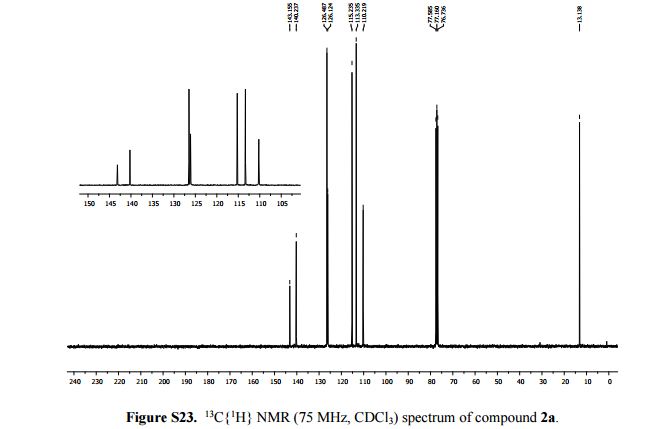
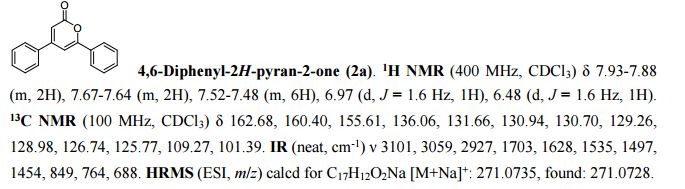
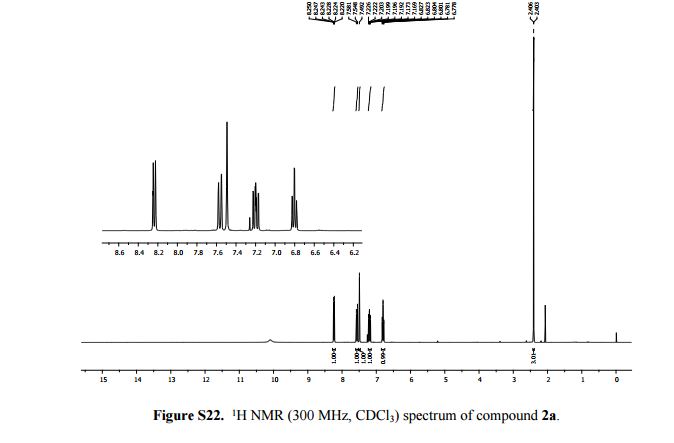
2-Methylimidazo[1,2-a]pyridine 2a:1 2-Amino-1-(2-propynyl)pyridinium bromide (1a) was reacted according to the general procedure (vide supra), affording the product as a colourless oil which solidifies under vacuum at room temperature. Yield: 0.13 g, 100% yield.
1H NMR (300 MHz, CDCl3): δ (ppm) 8.24 (dt, J = 6.6, 2.1, 0.9 Hz, 1H, pyH), 7.58 (d, J = 9.0 Hz, 1H, pyH), 7.49 (s, 1H, imH), 7.20 (m, 1H, pyH), 6.80 (td, J = 9.0, 6.6, 0.9 Hz, 1H, pyH), 2.41 (d, J = 0.9 Hz, 3H, CH3).
13C{1H} NMR (75 MHz, CDCl3): δ (ppm) 143.2, 140.2, 126.5, 126.1, 115.2, 113.3, 110.2, 13.1.
HR-MS (ESI+ ): m/z 133.0759 [C8H9N2] + , calcd. [M + H]+ 133.0760.
Anal. calcd. (%) for C8H8N2: C 72.70, H 6.10, N 21.10; found C 72.70, H 6.50, N 20.75.
Lit. data:1 1H NMR (500 MHz, DMSO-d6) 8.29 (s, 1H, CH), 7.59 – 7.03 (m, 4H, pyH), 1.21 (s, 3H, CH3).
13C NMR (125 MHz, DMSO-d6) 148.0, 140.0, 137.1, 130.8, 130.1, 116.2, 114.5, 34.1.
1 M. Bakherad, H. N. –Isfahani, A. Keivanloo, N. Doostmohammadi, Tetrahedron Lett. 2008, 49, 3819-3822
//////////