Balsalazide
| 80573-04-2; Colazal; Balsalazide Disodium; AC1NSFNR; P80AL8J7ZP; |
| Molecular Formula: | C17H15N3O6 |
|---|
| Molecular Weight: | 357.322 g/mol |
|---|
(3E)-3-[[4-(2-carboxyethylcarbamoyl)phenyl]hydrazinylidene]-6-oxocyclohexa-1,4-diene-1-carboxylic acid

DISODIUMDIHYDRATE
| CAS Number | 150399-21-6 |
|---|
| Weight | Average: 437.316
Monoisotopic: 437.08110308 |
|---|
| Chemical Formula | C17H17N3Na2O8 |
|---|
Balsalazide is an anti-inflammatory drug used in the treatment of
inflammatory bowel disease. It is sold under the brand names
Giazo,
Colazal in the US and
Colazide in the UK. It is also sold in generic form in the US by several generic manufacturers.
It is usually administered as the disodium salt. Balsalazide releases
mesalazine, also known as 5-aminosalicylic acid, or 5-ASA,
[1] in the large intestine. Its advantage over that drug in the treatment of
ulcerative colitis is believed to be the delivery of the active agent past the small intestine to the large intestine, the active site of ulcerative colitis.
Balsalazide is an anti-inflammatory drug used in the treatment of Inflammatory Bowel Disease. It is sold under the name "
Colazal" in the US and "
Colazide" in the UK. The chemical name is (E)-5-[[-4-(2-carboxyethyl) aminocarbonyl] phenyl]azo] -
2-hydroxybenzoic acid. It is usually administered as the
disodium salt.
Balsalazide releases
mesalazine, also known as
5-aminosalicylic acid, or
5-ASA, in the large intestine. Its advantage over that drug in the treatment of Ulcerative colitis is believed to be the delivery of the active agent past the small intestine to the large intestine, the active site of ulcerative colitis.
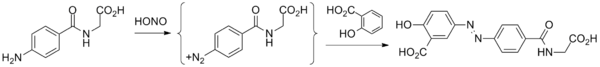
Balsalazide disodium and its complete synthesis was first disclosed by Chan[18] in 1983, assigned to Biorex Laboratories Limited, England, claiming product ‘Balsalazide’ and process of its preparation. The synthesis involves converting 4-nitrobenzoyl chloride (6) to 4- nitrobenzoyl-β-alanine (7), hydrogenating with Pd/C (5%) in ethanol and isolating by adding diethyl ether to produce 4-aminobenzoyl-β-alanine (8). Thereafter, 4-aminobenzoyl-β-alanine (8) was treated with hydrochloric acid and sodium nitrite to generate N-(4-diazoniumbenzoyl)- β-alanine hydrochloride salt (9) which was reacted at low temperature with disodium salicylate to furnish Balsalazide disodium insitu which was added to dilute hydrochloric acid at low temperature to produce Balsalazide (1) (Scheme-1.1). Thus obtained Balsalazide was recrystallized with hot ethanol and converted to pharmaceutically acceptable salt (disodium salt).
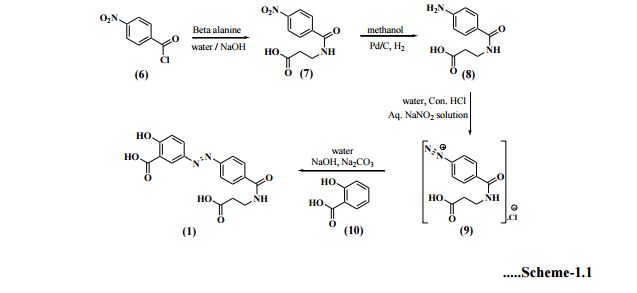
Optimization of this diazonium salt based process was performed by Huijun et al[19] and reported the preparation of the title compound in 64.6% overall yield. Zhenhau et al[20] have synthesized 1 from 4-nitrobenzoic acid (12) via chlorination, condensation, hydrogenation, diazotization, coupling and salt formation with overall yield 73%. Li et al[21] have given product in 73.9% total yield starting from 4-nitrobenzoyl chloride (6), where as Yuzhu et al[22] confirmed chemical structure of Balsalazide disodium by elemental analysis, UV, IR, 1H-NMR and ESI-MS etc. Shaojie et al[23] have also followed same process for its preparation. Yujie et al[24] synthesized 1 in this way; preparation of 4-nitrobenzoyl-β-alanine (7) under microwave irradiation of 420 W at 52oC for 10sec., reduction in ethyl acetate in the presence of Pd/C catalyst then diazotization, coupling and salt formation. Eckardt et al[25] have developed a process for the preparation of Balsalazide which comprises, conversion of 4-aminobenzoyl-β-alanine (8) to 4-ammoniumbenzoyl-β-alanine sulfonate salt using a sulfonic acid in water. This was treated with aq. sodium nitrite solution at low temperature to generate 4-diazoniumbenzoyl-β-alanine sulfonate salt (11) which was quenched with aq. disodium salicylate to furnish Balsalazide disodium solution. This was further acidified to allow isolation of 1 and then conversion to disodium salt (Scheme-1.2) in 76% yield.
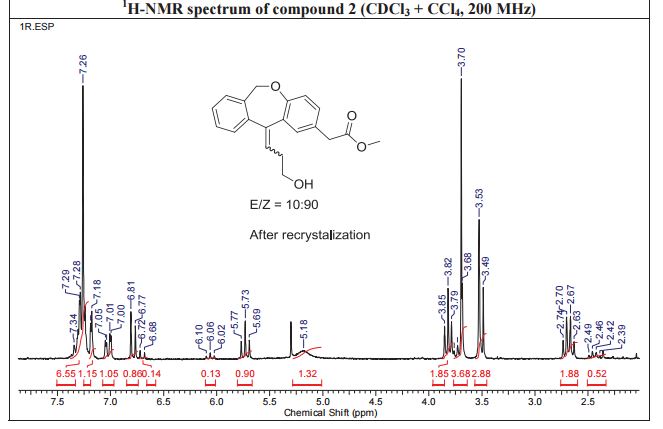
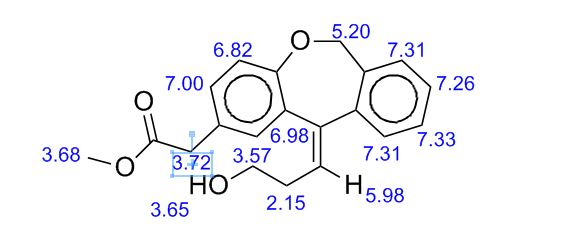
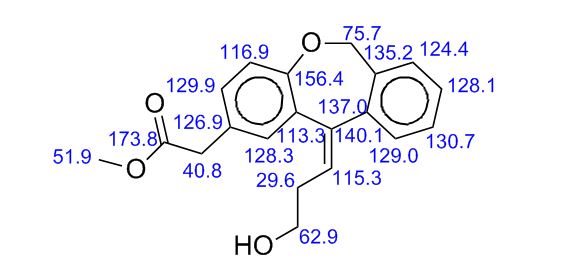
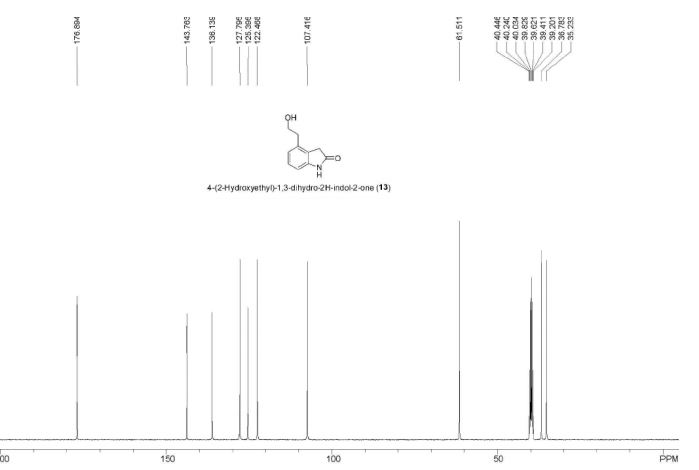
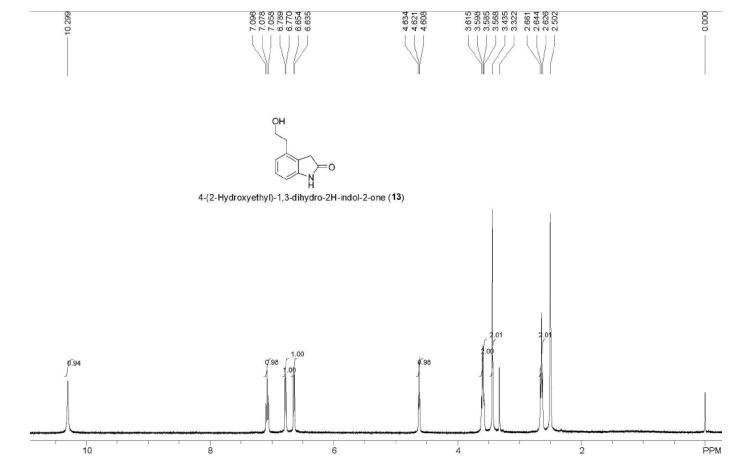
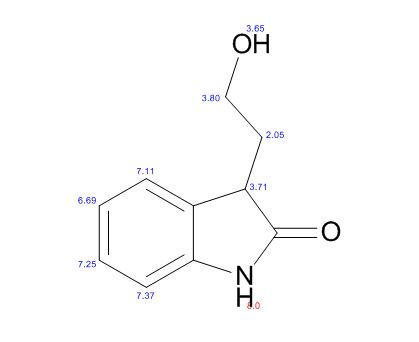
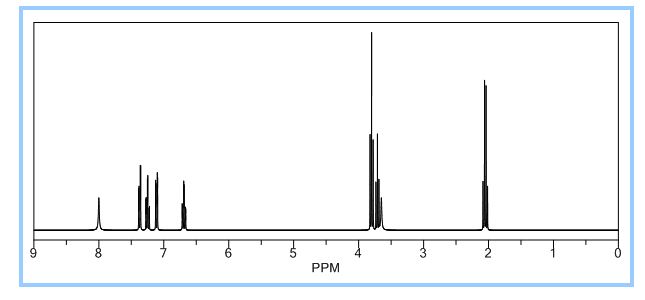
IR (KBr, cm-1 ): 3371 and 3039 (OH and NH), 1705 and 1699 (C=O), 1634 (C=O amide), 1590 and 1538 (C=C aromatic), 1464 and 1404 (aliphatic C-H), 1229 (C-N), 1073 (C-O), 773 and 738 (Ar-H out of plane bend). 1H NMR (DMSO-d6, 300 MHz, δ ppm): 2.54 (t, 2H), 3.50 (m, 2H), 6.95 (d, J = 8.8 Hz, 1H), 7.87 (d, J = 8.5 Hz, 2H), 8.02 (d, J = 8.5 Hz, 2H), 7.95 (dd, J = 8.8 Hz and 2.5 Hz, 1H), 8.34 (d, J = 2.5 Hz, 1H), 8.68 (t, J = 5.5 Hz, 1H), 12.12 (brs, 1H). MS m/z (ESI): 356 [(M-H)- ], Calculated; m/z 357.
Synthesis
Balsalazide synthesis: Biorex Laboratories,
GB 2080796 (1986).
- Starting material is 4-aminohippuric acid, obtained by coupling para-aminobenzoic acid and glycine.
- That product is then treated with nitrous acid to give the diazonium salt.
- Reaction of this species with salicylic acid proceeds at the position para to the phenol to give balsalazide.
Sodium balsalazide (Balsalazide sodium)
Brief background information
| Salt | ATC | Formula | MM | CAS |
|---|
| - | A07EC04 | C 17 H 13 N 3 Na 2 O 6 | 401.29 g / mol | 82101-18-6 |
| (E) is the free acid | A07EC04 | C 17 H 15 N 3 O 6 | 357.32 g / mol | 80573-04-2A |
Application
Classes substance
Synthesis Way
Trade names
| A country | Tradename | Manufacturer |
|---|
| United Kingdom | Kolazid | Shire |
| Italy | Balzid | Menarini |
| USA | Kolazal | Salix |
| Ukraine | no | no |
Formulations
PATENT
Balsalazide disodium (1) represents an effective gastrointestinal anti-inflammatory compound useful as a medicament for the treatment of diseases such as ulcerative colitis. It is delivered intact to the colon where it is cleaved by bacterial azoreduction thereby generating 5-aminosalicylic acid as the medicinally active component.
To date, relatively few patents or literature articles have dealt with the preparation of Balsalazide or the disodium salt. For instance, U.S. Pat. No. 4,412,992 (Biorex, 1983) is the first patent that we uncovered that claims the compound Balsalazide and a strategy of how to prepare it which strategy is depicted in Scheme 1.
Optimization of this diazonium-based process is detailed in Shan et al., Zhongguo Yaowu Huaxue Zazhi, 11, 110 (2001) and Shi et al., Zhongguo Yiyao Gongye Zazhi, 34, 537 (2003).
Problems arise with the above strategy and the optimization process.
It is well-documented in the literature, for instance in Thermochimica Acta, 225, 201-211 (1993), that diazonium salts can be involved in serious accidents in their use. A possible cause of some of the diazonium salt related accidents is that, for one reason or another, an intermediate material appeared in crystalline form in the vessel of the reaction. As a result, a potentially severe drawback of the above processes occurs. Since the intermediate hydrochloride salt of 4-aminobenzoyl-β-alanine has poor solubility in water, it may pose a safety-risk in the subsequent diazotation reaction.
Also, it is well-known that certain diazonium salts possess high mechanical and heat sensitivity and that their decomposition goes through the liberation of non-condensable nitrogen gas which results in the possibility of runaway reactions and explosions. Obviously this safety consideration becomes more pertinent upon further scale-up.
Therefore, for commercial production of Balsalazide disodium, there was a need to develop a scalable and intrinsically better process
Example 1 Batch Process
N-(4-Aminobenzoyl)-β-alanine (100 g) was suspended in water (1300 mL) and methanesulfonic acid (115.4 g) was added to this mixture. The mixture was cooled to 10° C. and a solution of sodium nitrite (34.46 g) in water (200 mL) was added at a rate such that the temperature stayed below 12° C. The mixture was stirred for 30 min and added to an ice-cold solution of salicylic acid (69.65 g), sodium hydroxide (40.35 g) and sodium carbonate (106.9 g) in 1 L water at 7-12° C. After 3 hours at 10° C., the mixture was heated to 60-65° C. and acidified to pH 4.0-4.5 by the addition of hydrochloric acid. After a further 3 hours at 60-65° C., the mixture was cooled to ambient temperature, filtered, washed with water and dried in vacuo to yield Balsalazide. Yield ca. 90%. Balsalazide was transformed into its disodium salt in ca. 85% yield by treatment with aqueous NaOH solution followed by crystallization from n-propanol/methanol.
1H-NMR (400 MHz; D2O): δ=8.04 ppm (s); 7.67 ppm (d; J=8.2 Hz); 7.62 ppm (d, J=9.2 Hz); 7.53 ppm (d; J=8.2 Hz); 6.84 ppm (d; J=8.9 Hz); 3.57 ppm (t, J=7.1 Hz); 2.53 ppm (t; J=7.2 Hz).
Example 2 Continuous Process
For the continuous operation, a conventional dual-head metering pump (Ratiomatic by FMI) was used to deliver the mesylate solution and the aqueous sodium nitrite solution. The schematic diagram shown in FIG. 4 represents a set-up used for the continuous process. The first pump-head was set at 13.9 g/min whereas the second was set at 2.1 g/min. These flow rates offered a residence time of 9.4 min. The yield of the coupled intermediate from this residence time was 93%. The working solutions were prepared as follow:
The mesylate solution was prepared by the addition into a 2 L 3-necked round bottom flask, of N-(4-aminobenzoyl) β-alanine (120 g) followed by of DI water (1560 g) and methanesulfonic acid (177.5 g) (Batch appearance: clear solution). The first pump-head was primed with this solution and the flow rate was adjusted to 13.9 g/min.
The sodium nitrite solution was prepared by dissolving of sodium nitrite (41.8 g) in of DI water (240 g) (Batch appearance: clear solution). The second pump-head was primed with this solution and the flow rate adjusted to 2.1 g/min.
The quenching solution (sodium salicylate) was made by adding salicylic acid (139.3 g) to DI water (900 g) followed by of sodium carbonate (106.9 g) and 50% aqueous sodium hydroxide (80 g).
The diazotation reaction was performed in a 500 ml jacketed flow reactor with a bottom drain valve. The drain valve was set at 16 g/min. For reactor start-up, the flow reactor was charged with 150 mL of DI water as a working volume and cooled to the reactions initial temperature of 0-5° C. Concomitantly, the additions of the mesylate and sodium nitrite solutions were started and the bottom valve of the flow reactor was opened. During the diazotization, the flow rate of both solutions remained fixed and the temperature was kept below 12° C. and at the end of additions the pumps were stopped while the remaining contents in the flow reactor were drained into the quenching salicylic acid solution. Analysis of the contents in the quenching reactor indicated no signs of uncoupled starting material (diazonium compound). The reactor contents were heated to 60-65° C. for 2-3 hrs before adjusting the pH to precipitate the coupling product. This provided 191.5 g of product.
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|
| US4412992 | Jul 8, 1981 | Nov 1, 1983 | Biorex Laboratories Limited | 2-Hydroxy-5-phenylazobenzoic acid derivatives and method of treating ulcerative colitis therewith |
| US6458776 * | Aug 29, 2001 | Oct 1, 2002 | Nobex Corporation | 5-ASA derivatives having anti-inflammatory and antibiotic activity and methods of treating diseases therewith |
| Reference |
|---|
| 1 | | Chai, et al., Huaxi Yaoxue Zazhi, Jiangsu Institute of Materia Medica, Nanjing, China, 2004, 19(6), 431-433. |
| 2 | | Shan, et al., Zhongguo Yaowu Huaxue Zazhi, Institute of Materia Medica, Peking Union Medical College, Beijing China, 2001, 11(2), 110-111. |
| 3 | | Shi, et al., Zhongguo Yiyao Gongya Zazhi, Shanghai Institute of Pharmaceutical Industry, Shanghai, China, 2003, 34(11), 537-538. |
| 4 | | Su, et al., Huaxue Gongye Yu Gongcheng (Tianjin, China), College of Chemistry and Chemical Eng., Donghua Univ., Shanghai, China, 2005, 22(4), 313-315. |
| 5 | | Ullrich, et al., Decomposition of aromataic diazonium compounds, Thermochimica Acta, 1993, 225, 201-211. |
References
References
- Jump up^ Kruis, W.; Schreiber, I.; Theuer, D.; Brandes, J. W.; Schütz, E.; Howaldt, S.; Krakamp, B.; Hämling, J.; Mönnikes, H.; Koop, I.; Stolte, M.; Pallant, D.; Ewald, U. (2001). "Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses". Gut. 49 (6): 783–789. doi:10.1136/gut.49.6.783. PMC 1728533
 . PMID 11709512.
. PMID 11709512.
| Patent ID | Patent Title | Submitted Date | Granted Date |
|---|
| US8232265 | Multi-functional ionic liquid compositions for overcoming polymorphism and imparting improved properties for active pharmaceutical, biological, nutritional, and energetic ingredients | 2007-04-26 | 2012-07-31 |
| US2011319267 | AROMATIC CARBOXYLIC ACID DERIVATIVES FOR TREATMENT AND PROPHYLAXIS OF GASTROINTESTINAL DISEASES INCLUDING COLON CANCERS | 2011-12-29 | |
| US2007213304 | Use of Aminosalicylates in Diarrhoea-Predominent Irritable Bowel Syndrome | 2007-09-13 | |
| US7119079 | Bioadhesive pharmaceutical compositions | 2004-07-22 | 2006-10-10 |
| US6699848 | Bioadhesive anti-inflammatory pharmaceutical compositions | | 2004-03-02 |
CLICK ON IMAGE
Title: Balsalazide
CAS Registry Number: 80573-04-2
CAS Name: 5-[(1E)-[4-[[(2-Carboxyethyl)amino]carbonyl]phenyl]azo]-2-hydroxybenzoic acid
Additional Names: (E)-5-[[p-[(2-carboxyethyl)carbamoyl]phenyl]azo]-2-salicylic acid
Molecular Formula: C17H15N3O6
Molecular Weight: 357.32
Percent Composition: C 57.14%, H 4.23%, N 11.76%, O 26.87%
Literature References: Analog of sulfasalazine, q.v. Prodrug of 5-aminosalicylic acid where carrier molecule is 4-aminobenzoyl-b-alanine. Prepn: R. P. K. Chan, GB 2080796; idem, US 4412992 (1982, 1983 both to Biorex). Toxicology study and clinical metabolism: idem et al., Dig. Dis. Sci. 28, 609 (1983). Review of pharmacology and clinical efficacy in ulcerative colitis: A. Prakash, C. M. Spencer, Drugs 56, 83 (1998).
Properties: Crystals from hot ethanol, mp 254-255°.
Melting point: mp 254-255°
Derivative Type: Disodium salt dihydrate
CAS Registry Number: 150399-21-6; 82101-18-6 (anhydrous)
Manufacturers' Codes: BX-661A
Trademarks: Colazal (Salix); Colazide (Shire)
Molecular Formula: C17H13N3Na2O6.2H2O
Molecular Weight: 437.31
Percent Composition: C 46.69%, H 3.92%, N 9.61%, Na 10.51%, O 29.27%
Properties: Orange to yellow microcrystalline powder, mp >350°. Nonhygroscopic. Freely sol in water, isotonic saline; sparingly sol in methanol, ethanol. Practically insol in organic solvents.
Melting point: mp >350°
Therap-Cat: Anti-inflammatory (gastrointestinal).
Keywords: Anti-inflammatory (Gastrointestinal); Anti-inflammatory (Nonsteroidal); Salicylic Acid Derivatives.
//////
O=C(O)c1cc(ccc1O)/N=N/c2ccc(cc2)C(=O)NCCC(O)=O
O.O.[Na+].[Na+].OC1=CC=C(C=C1C([O-])=O)\N=N\C1=CC=C(C=C1)C(=O)NCCC([O-])=O
|
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.


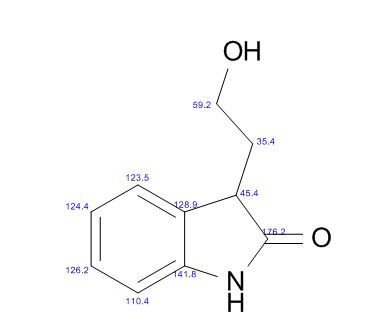

 DISODIUMDIHYDRATE
DISODIUMDIHYDRATE