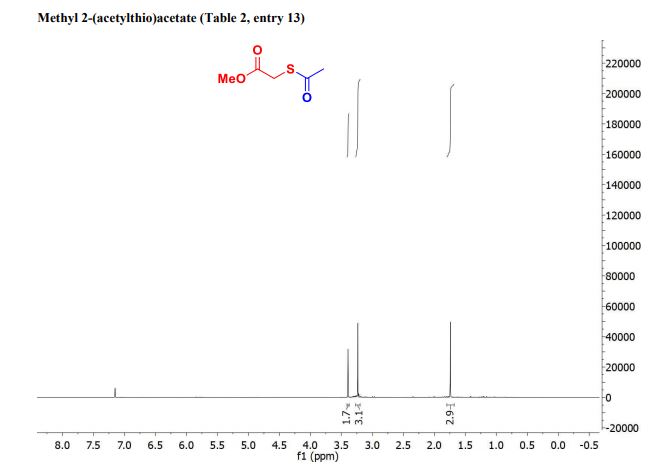
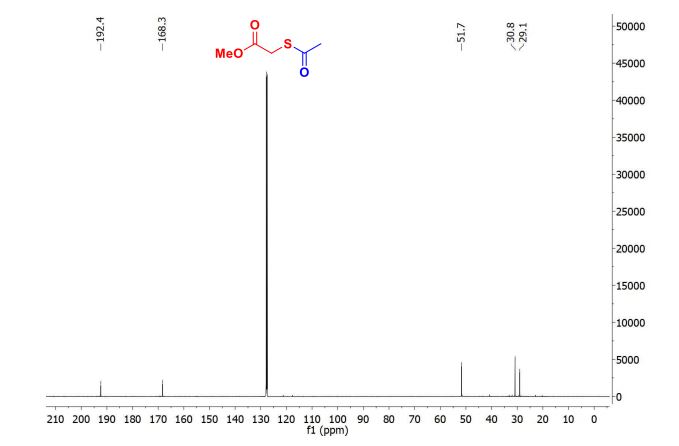
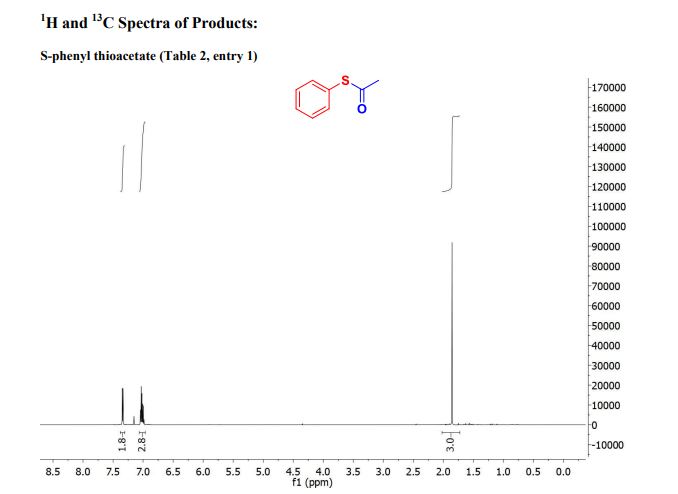
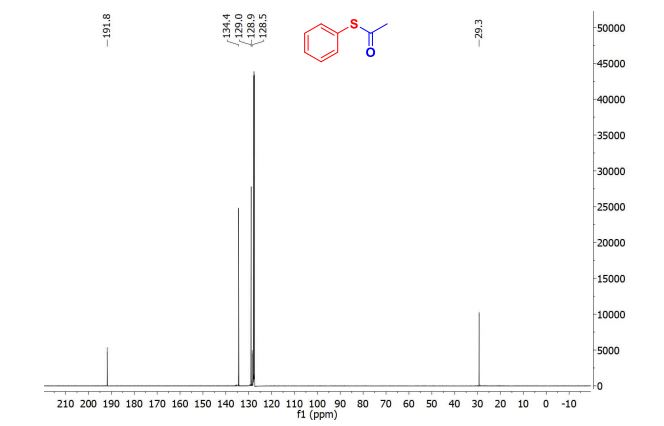
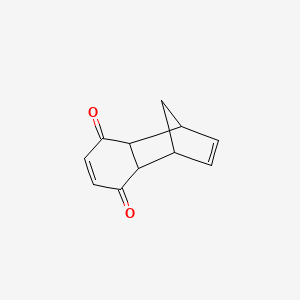
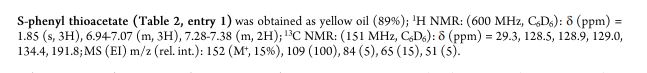| Chemical Names: | Cyclopentadienebenzoquinone; 1200-89-1; 1,4,4a,8a-Tetrahydro-1,4-methanonaphthalene-5,8-dione; 1,4-Methanonaphthalene-5,8-dione, 1,4,4a,8a-tetrahydro-; NSC 25329; 1,4,4a,8a-Tetrahydro-endo-1,4-methanonaphthalene-5,8-dione |
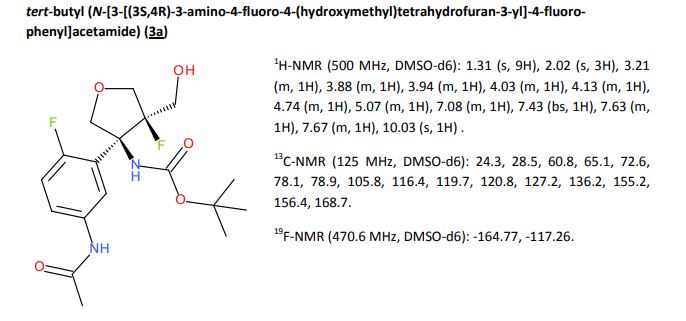
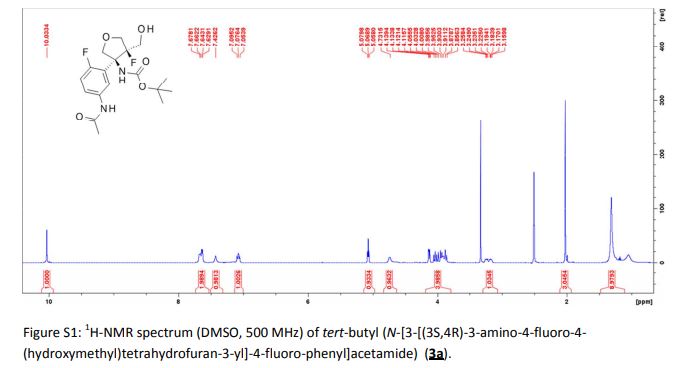
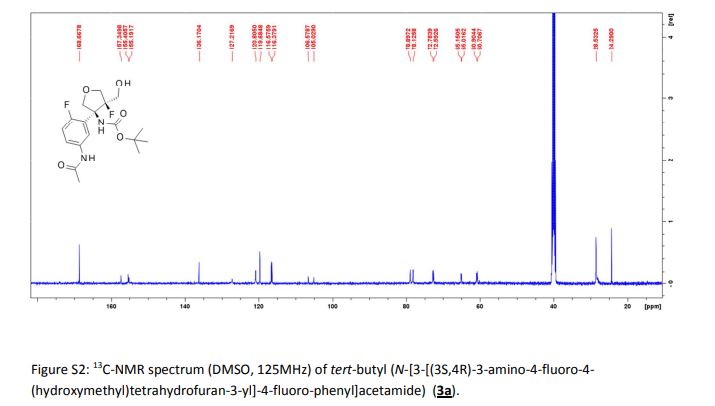
|---|
| Molecular Formula: | C11H10O2 |
|---|
| Molecular Weight: | 174.199 g/mol |
|---|
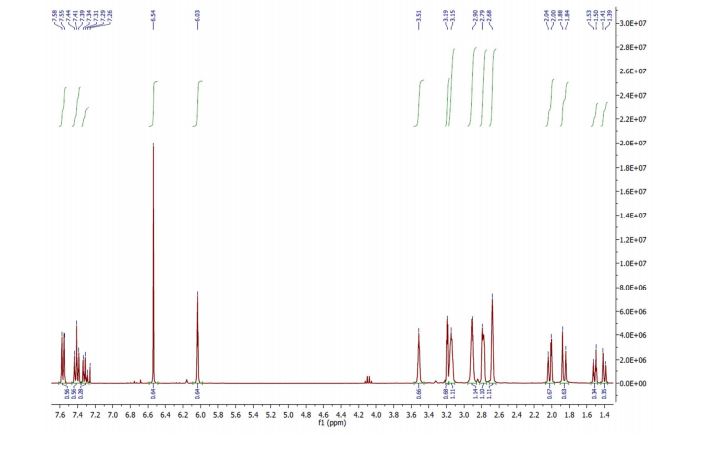
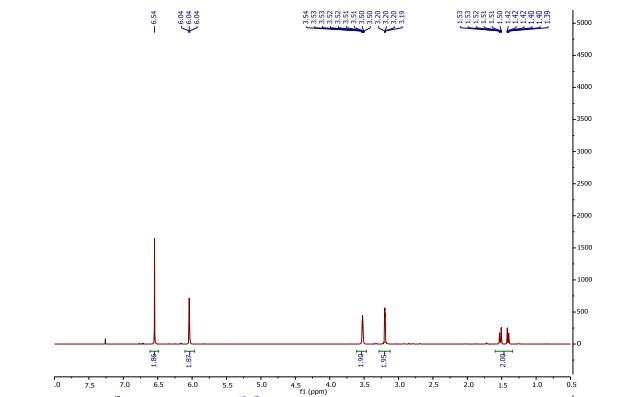
1H NMR (CDCl3, 400 MHz) δ = 1.41 (dt, J = 8.8, 1.5 Hz, 1H), 1.52 (dt, J = 8.8, 1.8 Hz, 1H), 3.20 (dd, J = 2.5, 1.5 Hz, 2H), 3.52 (m, 2H), 6.04 (t, J = 1.9 Hz, 2H), 6.54 (s, 2H).
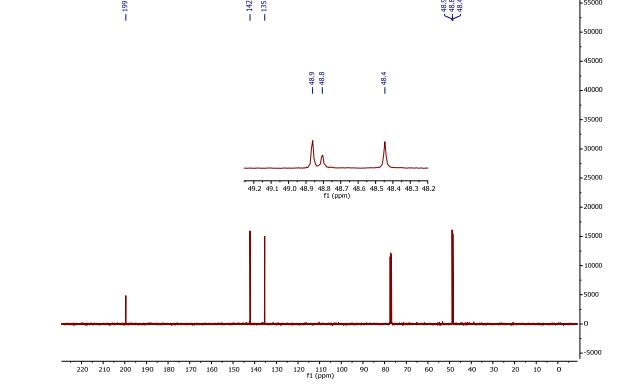
13C NMR (CDCl3, 100 MHz) δ = 48.4 (2 × CH), 48.8 (CH), 48.9 (2 × CH), 135.4 (2 × CH), 142.1 (2 × CH), 199.5 (2 × CO)
O=C3C=CC(=O)C2C3C1C=CC2C1
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00037

We report the use of a simple rotary evaporator as a semi-continuous UV photochemical reactor. By generation of a thin film from the rotation of a flask, better light penetration is achieved, and in this work we used high-power Hg lamps to enable the direct irradiation of molecules with UV light. The intramolecular [2 + 2] photocycloaddition of Cookson’s dione and the intermolecular [2 + 2] photocycloaddition of maleimide with 1-hexyne were used as test reactions to examine the effectiveness of this reactor. High productivities, equivalent to 210 g h
–1, were obtained for the simple intramolecular reaction, demonstrating the scalability of the reactor. The effects of flask size, reaction mixture volume, and use of borosilicate or quartz glassware were also investigated.
Cookson’s Dione
1H NMR (CDCl3, 300 MHz) δ = 1.86 (d, J = 11.3, 1H), 2.02 (d, J = 11.3, 1H), 2.68 (m, 2H), 2.79 (m, 2H), 2.90 (m, 2H), 3.15 (m, 2H).
References 1. Clark, C. A.; Lee, D. S.; Pickering, S. J.; Poliakoff, M.; George, M. W. Org. Process Res. Dev. 2016, 20, 1792-1798. 2. Elliott, L. D.; Knowles, J. P.; Koovits, P. J.; Maskill, K. G.; Ralph, M. J.; Lejeune, G.; Edwards, L. J.; Robinson, R. I.; Clemens, I. R.; Cox, B.; Pascoe, D. D.; Koch, G.; Eberle, M.; Berry, M. B.; Booker-Milburn, K. I. Chem. Eur. J. 2014, 20, 15226-15232. 3. Marchand, A. P.; Allen, R. W. J. Org. Chem. 1974, 39, 1596-1596. 4. Elliott, L. D.; Berry, M.; Harji, B.; Klauber, D.; Leonard, J.; Booker-Milburn, K. I. Org. Process Res. Dev. 2016, 20, 1806-1811. 5. Hook, B. D. A.; Dohle, W.; Hirst, P. R.; Pickworth, M.; Berry, M. B.; Booker-Milburn, K. I. J Org Chem 2005, 70, 7558-7564.
https://orgspectroscopyint.blogspot.com/search/label/Cookson’s%20Dione
Oct 3, 2016 - Cookson's Dione 125 W Batch Reaction A solution of Diels Alder adduct 3[3] (2.61 g, 15 mmol) in degassed EtOAc (150 ml) was irradiated with a pre-warmed 125 W medium pressure mercury lamp in a 150 ml batch reactor equipped with a. Pyrex immersion well for 15 min. The solvent was removed in ..
///////////

 We report the use of a simple rotary evaporator as a semi-continuous UV photochemical reactor. By generation of a thin film from the rotation of a flask, better light penetration is achieved, and in this work we used high-power Hg lamps to enable the direct irradiation of molecules with UV light. The intramolecular [2 + 2] photocycloaddition of Cookson’s dione and the intermolecular [2 + 2] photocycloaddition of maleimide with 1-hexyne were used as test reactions to examine the effectiveness of this reactor. High productivities, equivalent to 210 g h
We report the use of a simple rotary evaporator as a semi-continuous UV photochemical reactor. By generation of a thin film from the rotation of a flask, better light penetration is achieved, and in this work we used high-power Hg lamps to enable the direct irradiation of molecules with UV light. The intramolecular [2 + 2] photocycloaddition of Cookson’s dione and the intermolecular [2 + 2] photocycloaddition of maleimide with 1-hexyne were used as test reactions to examine the effectiveness of this reactor. High productivities, equivalent to 210 g h