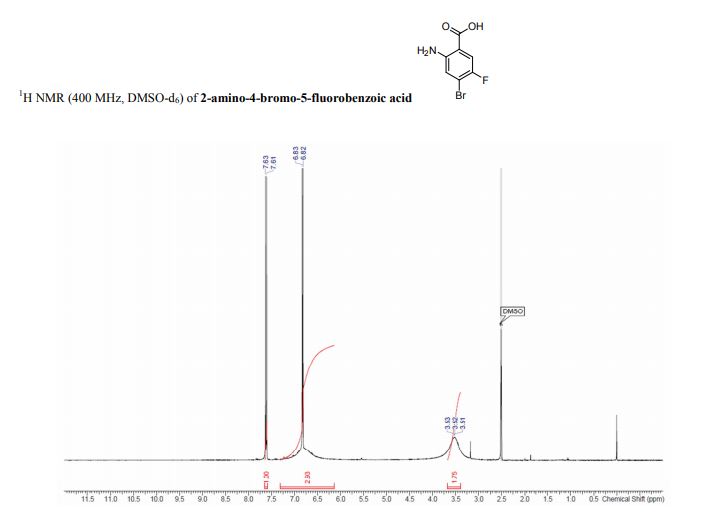
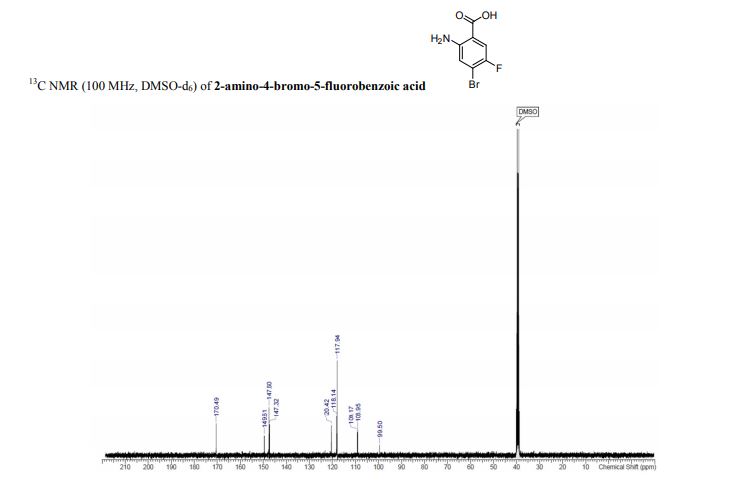
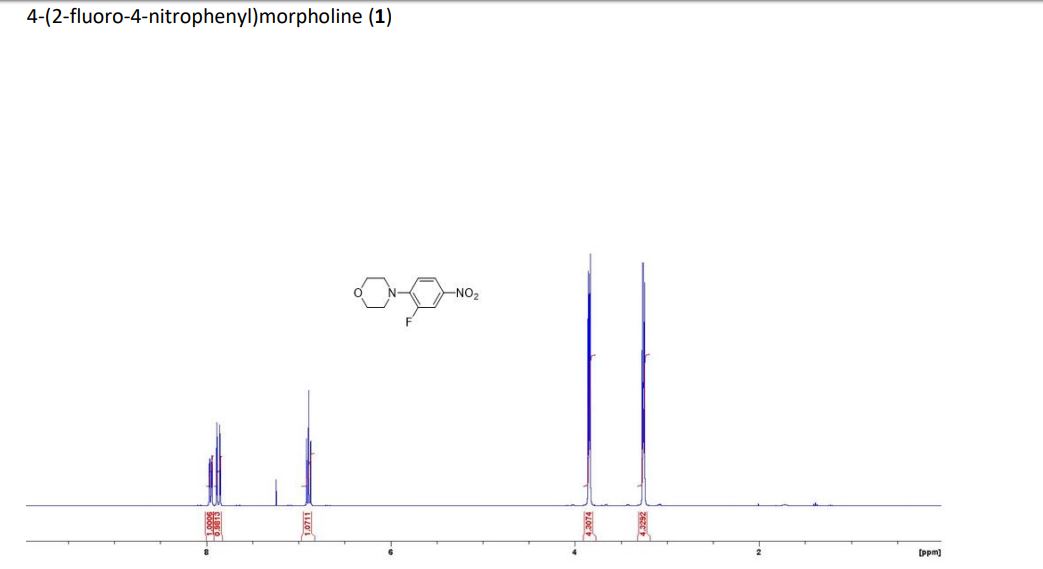
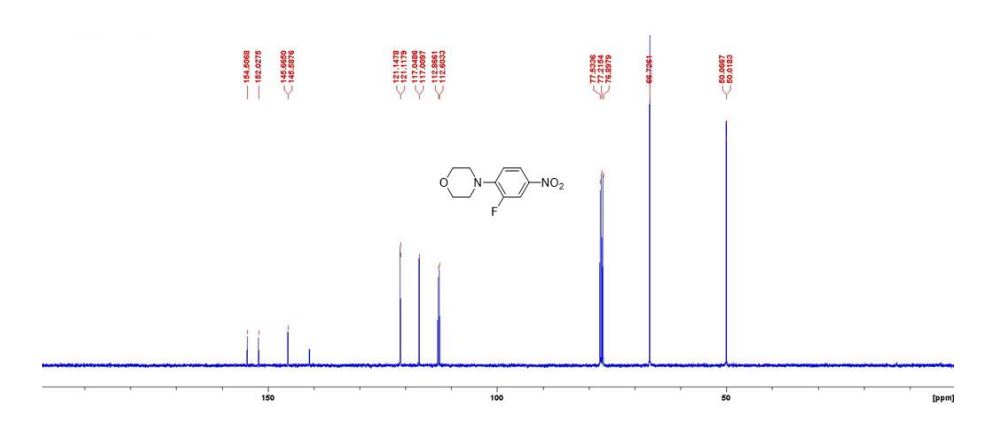
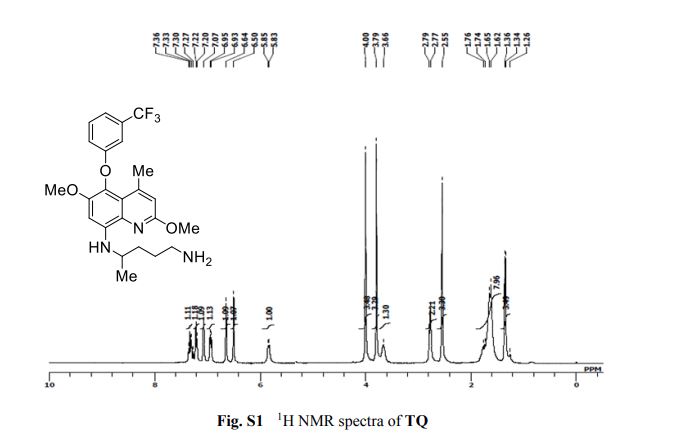
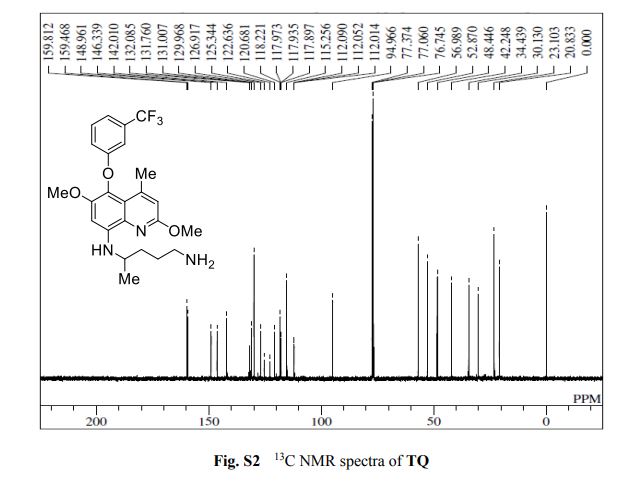
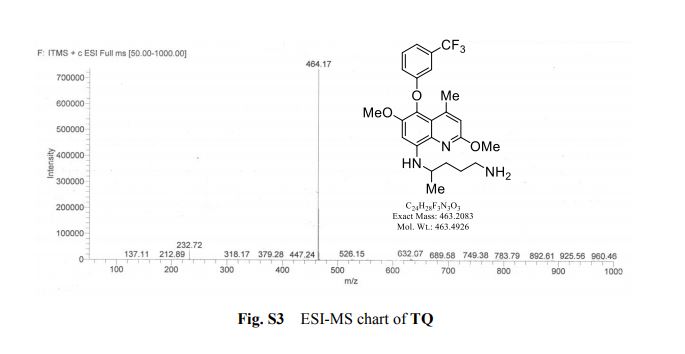
2-amino-4-bromo-5-fluorobenzoic acid as a white to off-white crystalline solid
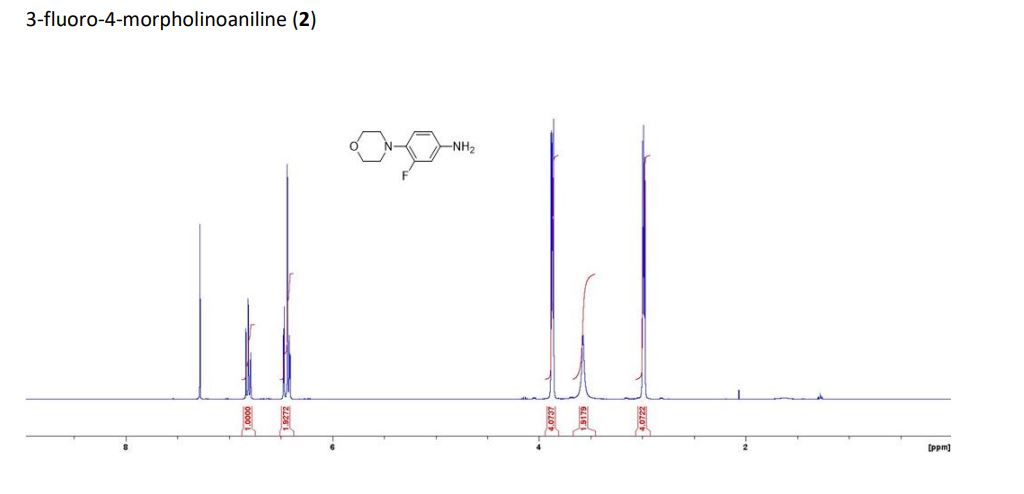
1H NMR (400 MHz, DMSO-d6) δ 7.62 (d, J=9.6 Hz, 1H), 7.21-6.5 (m, 3H), 3.8- 3.3 (br s, 1H).
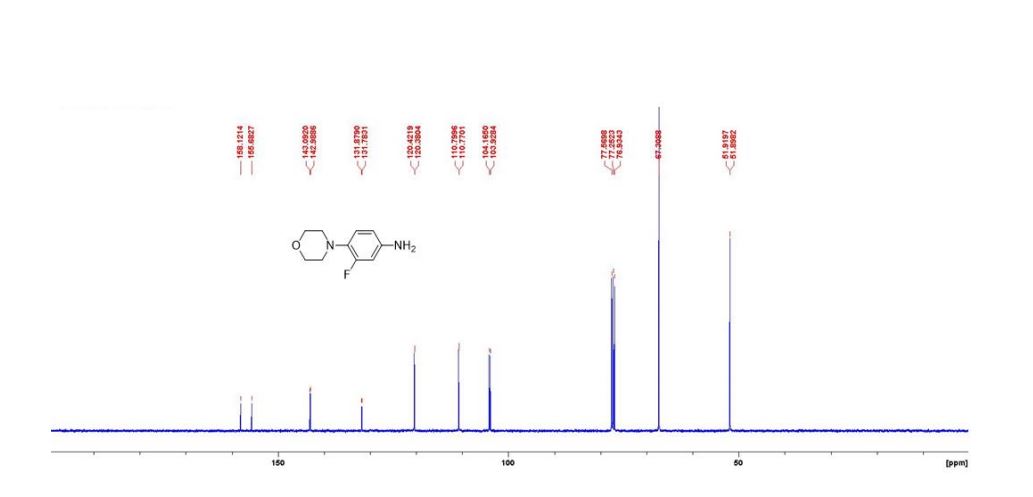
13C NMR (100 MHz, DMSO-d6) δ 170.5, 149.6, 147.6, 147.3, 120.4, 118.1, 118.0, 109.2, 109.0, 99.5.
mp >250 °C. IR (neat) 3494, 3351, 3053, 3038, 1521, 774 cm-1;
HRMS (ESI) m/z: calcd for C7H5BrFNO2 [M+H]+ 233.9560, found 233.9551.
Org. Lett., 2018, 20 (13), pp 3736–3740
DOI: 10.1021/acs.orglett.8b01218
////////////