(R)-3-Chloro-2-hydroxypropyl-4-methoxybenzoate 10
To a stirred solution of (R)-3-chloro-1,2-propanediol (6.61 g, 59.8 mmol) in CH2Cl2 (120 mL) was added imidazole (4.07 g, 59.8 mmol). After the reaction mixture had cooled to 0 °C, p-methoxybenzoyl chloride (10.2 g, 59.8 mmol) in CH2Cl2 (10 mL) was added dropwise via addition funnel. The resulting solution was allowed to warm to room temperature and stirred until complete consumption of starting material by thin layer chromatography (TLC). The mixture was poured into saturated aq NH4Cl (150 mL), and the aqueous layer was extracted with CH2Cl2 (3 × 100 mL). The combined organic extracts were dried (MgSO4), filtered, and concentrated in vacuo to provide chlorohydrin 10as a clear, viscous oil (11.43 g, 78%) that was used without further purification. Rf 0.3 (20% EtOAc/hexanes); ee >99% as determined by chiral SFC (see the Supporting Information);
1H NMR (500 MHz, CDCl3) δ 8.07−7.94 (m, 2H), 7.00−6.88 (m, 2H), 4.46 (d, J = 5.1 Hz, 2H), 4.22 (dd, J = 10.6, 5.3 Hz, 1H), 3.88 (s, 3H), 3.78−3.64 (m, 2H), 2.73 (d, J = 5.6 Hz, 1H);
13C NMR (126 MHz, CDCl3) δ 166.7, 163.9, 132.1, 121.9, 114.0, 70.1, 65.7, 55.7, 46.3;
IR [CH2Cl2 solution] νmax (cm−1) 3454, 2959, 2889, 1713, 1606, 1512, 1259, 1170, 1104, 1028, 848, 770, 697, 614;
HRMS (ESI-TOF) calcd for C11H13ClO4 (M)+244.0573, found 244.0501.
https://pubs.acs.org/doi/full/10.1021/jo1015807
/////////////////
https://pubs.acs.org/doi/suppl/10.1021/jo1015807/suppl_file/jo1015807_si_001.pdf
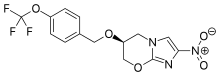


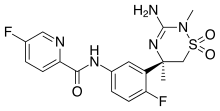
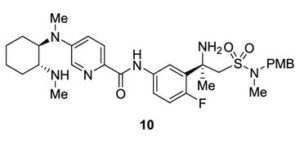
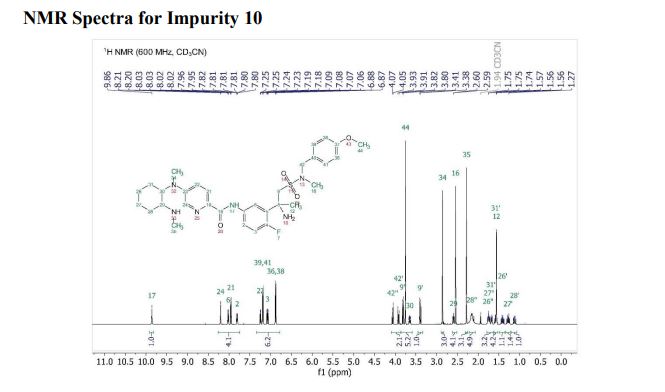






 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..