http://newdrugapprovals.org/2013/04/07/drug-spotlight-celecoxib-from-g-d-searle-company/
Celecoxib extraction
Celecoxib was extracted from Celebra™ 100
mg and 200 mg capsules. Ten capsules were
crushed to fine powder, using agate mortar and
pestle, transferred to a 50 ml volumetric flask
and diluted to volume with methanol. The solution
was shaked for 5 min and filtered. The
residue was recrystallized from acetonitrile after
evaporation of the solvent in a water bath at 50
°C, under a stream of nitrogen.
Thin layer Chromatography (TLC)
In this procedure it was used silica gel 60
F254 plates (20 x 20 cm) with a thickness of 0.25
mm. All plates used were commercially prepared
by MERCK (lot # 040422153). The mobile
phase used to develop the system consisted of
chloroform-ethyl acetate-ether (10:5:1, v/v/v).
Just in order to verify the selectivity of the
proposed system, another COX-2 inhibitor (rofecoxib)
with similar properties was used. 20 µl of
celecoxib reference substance and extracted
from tablets and rofecoxib solutions (50 µg.ml–1)
were spotted on the TLC plates, and transferred
to a developing tank containing the mobile
phase. The plate was then examined under UV
light (254 and 365 nm).
Thin-layer chromatography
One of the most effective screening methods
is the thin-layer chromatography (TLC), which is
the simplest of all the widely used chromatographic
methods to perform. In the determination
of this method, different chromatography
systems were tested and analyzed according to
the classification proposed by Moffat 13, which
divide the drugs in three categories: acid, basic
and neutrals based on their polarity and acid
characteristics. This procedure is important in
order to increase the information obtained only
by changing the mobile phase, and thus resulting
in a significant change in selectivity.
These chromatographic systems consisted of
mixtures of the following solvents: chloroformacetonitrile,
ethyl acetate, acetone and methanol,
in different concentrations. Nevertheless, all
the mobile phases tested were not adequate for
the proper identification of the drug. With the
objective to develop more reliable chromatographic
method, a modification in the system
tested was proposed, in order to improve the
chromatographic resolution. The mobile phase
used consisted of chloroform-ethyl acetate-ether
(10:5:1 v/v/v). The system was chosen due to its
sensitivity, simplicity and efficacy
The preference to use commercially prepared
silica gel plates was due to its durability
and homogeneity of the absorbent layer.
A sample solution of the working standard
and the drug sample (50 (µg ml–1) were spotted
onto a silica gel plate with a micropipette and
the chromatogram was developed by placing
the plate in a tank containing the mobile phase.
Following development the individual solute
spots were identified under UV lamp (254 and
365 nm). The spots in the drug sample and the
working standard presented similar Rf values.
The Rf value obtained for drug sample and the
working standard were 0.45 and for rofecoxib
0.32.
NMR
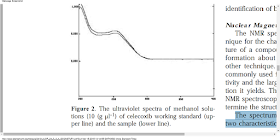
The spectrum shown in Figure 3 possesses
two characteristic sharp singlet peaks at 2.3 and3.3 ppm that belong to the methyl and sulfonamide
protons of celecoxib. The spectrum also
reveals peaks from 7.3 to 7.9, which is due to
the protons of aromatic groups. The characteristics
peaks from the working standard agree well
with those observed in the samples, considering
the fact that a constant shift is observed in all
peaks 11, 12.
UV
IR
1150 - 1350 S = O stretching
(sulfonamide group)
1550 - 1600 N - H stretching
3300 - 3500 NH2 stretching

1H NMR PREDICT

13 C NMR

(4) (a) Matsuo, M.; Tsuji, K.; Konishi, N.; Nakamura, K. EP patent
0,418,845, A1, 1990. (b) Matsuo, M.; Tsuji, K.; Konishi, N.; Ogino,
T. EP patent 0,554,829, A1, 1993. (c) Nishiwaki, T. Bull. Chem. Soc.
Jpn. 1969, 42, 3024. (d) Soliman, R.; Feid-allah, H. J. Pharm. Sci.
1980, 70, 602. (e) Wright, J. B.; Dulin, W. E.; Markillie, J. H. J. Med.
Chem. 1963, 7, 102. (f) Habeeb, A. G.; Rao, P. N. P.; Knaus, E. E.
J. Med. Chem. 2001, 44, 3039. (g) Szabo, G.; Fischer, J.; Kis-Varga,
A.; Gyires, K. J. Med. Chem. 2008, 51, 142. (h) Oh, L. M. Tetrehedron
Lett. 2006, 47, 7943.
(5) Talley, J. J.; Penning, T. D.; Collins, P. W.; Rogier, D. J.; Malecha,
J. W.; Miyashiro, J. M.; Bertenshaw, S. R.; Khanna, I. K.; Graneto,
M. J.; Rogers, R. S.; Carter, J. S. US patent 5,466,823, 1995.
(6) Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins,
P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.;
Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson,
G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.;
Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson,
P. C. J. Med. Chem. 1997, 40, 1347.
7 Talley, J. J.; Penning, T. D.; Collins, P. W.; Rogier, D. J.; Malecha,
J. W.; Miyashiro, J. M.; Bertenshaw, S. R.; Khanna, I. K.; Graneto,
M. J.; Rogers, R. S.; Carter, J. S.; Docter, S. H.; Yu, S. S. US patent
6,586,603, B1, 2003.
(8) (a) O′ Shea, P. ; Tillyer, R. D. ; Wang, X. ; Clas, S. D. ; Dalton, C.
US patent 6,150,534, 2000. (b) O′ Shea, P. ; Tillyer, R. D. ; Wang,
X. ; Clas, S. D. ; Dalton, C. US patent 6,232,472, 2001.
(9) (a) Zhi, B. ; Newaz, M. US patent 5,,892,053, 1999. (b) Zhi, B. ; Newaz,
M. US patent 5,910,597, 1999.
10(a) Letendre, L. J.; McGhee, W. D.; Snoddy, C.; Klemm, G.; Graud,
H. T. US patent 7,141,678, 2006. (b) Letendre, L. J.; McGhee, W. D.;
Snoddy, C.; Klemm, G.; Graud, H. T. US patent 2007/0,004, 924 A1,
2007.
(11) ICH harmonized tripartite guideline, Impurities in New Drug Substances
Q3A (R2), current step 4 version dated 25 October2006.
(12) Ahlstrom, M. M.; Ridderstrom, M.; Zamora, I.; Luthman, K. J. Med.
Chem. 2007, 50, 4444. (13) Soliman, R. J. Med. Chem. 1979, 22, 321.
SYNTHESIS

+


GIVES........CELECOXIB
 EUCLISES PHARMACEUTICALS, INC.; MARTINEZ, Eduardo, J.; TALLEY, John, J.; JEROME, Kevin, D.; BOEHM, Terri, L. Patent: WO2014/12074 A2, 2014 ; Location in patent: Paragraph 00209; 00210
EUCLISES PHARMACEUTICALS, INC.; MARTINEZ, Eduardo, J.; TALLEY, John, J.; JEROME, Kevin, D.; BOEHM, Terri, L. Patent: WO2014/12074 A2, 2014 ; Location in patent: Paragraph 00209; 00210
2

+



+

Reddy, Anumula Raghupathi; Sampath, Alla; Goverdhan, Gilla; Yakambaram, Bojja; Mukkanti, Kagga; Reddy, Padi Pratap Organic Process Research and Development, 2009 , vol. 13, # 1 p. 98 - 101
3
Prabhakaran, Jaya; Underwood, Mark D.; Parsey, Ramin V.; Arango, Victoria; Majo, Vattoly J.; Simpson, Norman R.; Van Heertum, Ronald; Mann, J. John; Kumar, J.S. Dileep Bioorganic and Medicinal Chemistry, 2007 , vol. 15, # 4 p. 1802 -
4
Li, Feng; Nie, Jing; Sun, Long; Zheng, Yan; Ma, Jun-An Angewandte Chemie - International Edition, 2013 , vol. 52, # 24 p. 6255 - 6258
5
Synlett, , vol. 1997, # 4 p. 375 - 377
6Tetrahedron Letters, , vol. 52, # 45 p. 6000 - 6002
7
Bioorganic and Medicinal Chemistry Letters, , vol. 21, # 22 p. 6636 - 6640

COCK WILL TEACH YOU NMR

COCK SAYS MOM CAN TEACH YOU NMR

Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com
 29.2 min
29.2 min 30.9 min
30.9 min
 COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
No comments:
Post a Comment