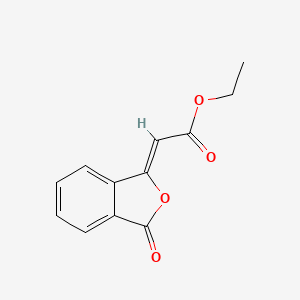
 ethyl (2Z)-2-(3-oxo-2-benzofuran-1-ylidene)acetate
218.2054 g/mol, C12H10O4
ethyl (2Z)-2-(3-oxo-2-benzofuran-1-ylidene)acetate
218.2054 g/mol, C12H10O4
Ethyl (Z)-3-phthalidylideneacetate;
[3-Oxoisobenzofuran-1(3H)-ylidene]acetic acid ethyl ester;
[(Z)-3-Oxoisobenzofuran-1(3H)-ylidene]acetic acid ethyl ester;
Synthesis of ethyl 2-(3-oxo-1,3-dihydro-1-isobenzofurany liden)acetate (2) A solution of phthalic anhydride (1.0 equiv.) and ethyl 2- (1,1,1-triphenyl-5 -phosphanylidene)acetate (1.1 equiv.) in 300 ml of dichloromethane (DCM) was refluxed for 3 hr. DCM was removed by vacuum at 40-50 o C. 2×150 ml of hexane was added to the resulting sticky solid, stirred for 10 min and the un-reacted 2-(1,1,1-triphenyl-5 -phosphanylidene)acetate was removed by filtration. The organic solvent was removed under vacuum and the resulting crude semisolid was taken to next step without further purification. Yield: 84%.
1 H-NMR CDCl3; (ppm): 1.1 (t, 3H), 4.2 (q, 2H), 6.0 (s, 1H), 7.6 (t, 1H), 7.7 (t, 1H), 7.8 (d, 1H), 8.9 (d, 1H). S
Medicinal Chemistry, 2009, Vol. 5, No. 5,
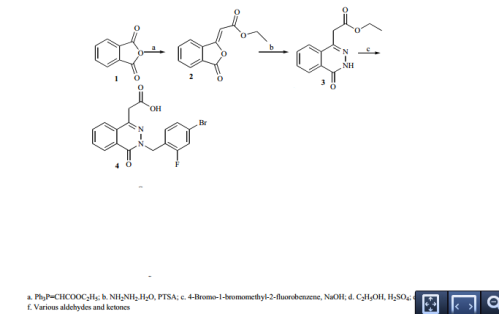 Method for producing alkyl 3-phthalidylideneacetate [US5508446]
Method for producing alkyl 3-phthalidylideneacetate [US5508446]
//////////
O=C(OCC)/C=C1\OC(=O)c2ccccc12
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
Join me on Linkedin

Join me on Facebook
 FACEBOOK
FACEBOOK
Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com
take a tour





























///////


 amcrasto@gmail.com
amcrasto@gmail.com





No comments:
Post a Comment