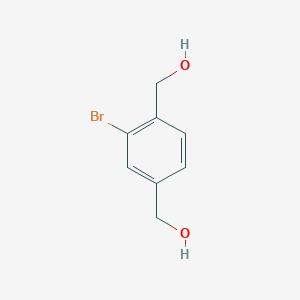
(2-bromo-4-hydroxymethylphenyl)methanol;
CAS 89980-92-7;
2-Bromo-1,4-benzenedimethanol;
| Molecular Formula: | C8H9BrO2 |
|---|---|
| Molecular Weight: | 217.05986 g/mol |
(2-Bromo-4-hydroxymethylphenyl)methanol (3).
To a solution of commercially available 2-bromoterephthalic acid (2) (575 g, 2.34 mol) in THF (5.75 L), a THF solution of BH3 (1.0 M, 5.86 L) was added at 0 °C dropwise for 2.5 h, and the mixture was stirred for 1 h at 0 °C. The mixture was gradually warmed up to 35 °C over 3.5 h. The reaction mixture was cooled to 0 °C and quenched by dropwise addition of MeOH (1.15 L) over 30 min. Then, the mixture was concentrated in vacuo. The residue was dissolved in MeOH (1.72 L), and then water (10.3 L) was added; the mixture was then stirred at 0 °C for 30 min. The off-white solid was filtered and washed with water (1.15 L × 3) and heptanes (2.30 L) to obtain 3 (426 g, 84%) as a white crystal;
mp 108–109 °C;
1H NMR (400 MHz, DMSO-d6) δ: 4.47 (2H, d, J = 5.6 Hz), 4.49 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 5.6 Hz), 5.39 (1H, t, J = 5.4 Hz), 7.31 (1H, d, J = 7.8 Hz), 7.47 (1H, d, J = 7.8 Hz), 7.48–7.49 (1H, m);
13C NMR (100 MHz, DMSO-d6) δ: 61.9, 62.5, 120.8, 125.5, 127.9, 129.7, 139.1, 143.4;
HRMS (EI) calcd for C8H9BrO2 [M]+ 215.9786, found 215.9787.
1H NMR (400 MHz, DMSO-d6) δ: 4.47 (2H, d, J = 5.6 Hz), 4.49 (2H, d, J = 5.4 Hz), 5.29 (1H, t, J = 5.6 Hz), 5.39 (1H, t, J = 5.4 Hz), 7.31 (1H, d, J = 7.8 Hz), 7.47 (1H, d, J = 7.8 Hz), 7.48–7.49 (1H, m);
13C NMR
13C NMR (100 MHz, DMSO-d6) δ: 61.9, 62.5, 120.8, 125.5, 127.9, 129.7, 139.1, 143.4;
MASS PREDICT
1H/13C PREDICT
J. Org. Chem., 2016, 81 (5), pp 2148–2153
DOI: 10.1021/acs.joc.5b02734








No comments:
Post a Comment