Vote

Masitinib
Masitinib; 790299-79-5; Masivet; AB1010; AB-1010;
TARGET:KIT (a stem cell factor, also called c-KIT) receptor as well as select other tyrosine kinases
COMMERCIAL:Under development by AB Science..
Ab Science
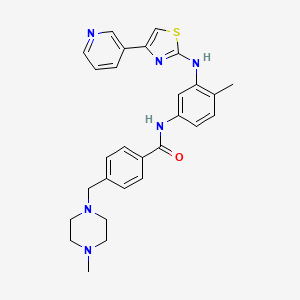
4-((4-Methylpiperazin-1-yl)methyl)-N-(4-methyl-3-((4-(pyridin-3-yl)-1,3-thiazol-2-yl)amino)phenyl)benzamide
AB 1010
UNII-M59NC4E26P
4-((4-Methylpiperazin-1-yl)methyl)-N-(4-methyl-3-((4-(pyridin-3-yl)-1,3-thiazol-2-yl)amino)phenyl)benzamide
Regulatory and Commercial Status
HIGHEST STATUS ACHIEVED (FOR ANY CONDITION):
Marketing Authorization Application for the
treatment of pancreatic cancer has been filed with the European
Medicines Agency (16 October 2012)
Marketing Authorization Application for the
conditional approval in the treatment of pancreatic cancer has been
accepted by the European Medicines Agency (30 October 2012)
Masitinib is a
tyrosine-kinase inhibitor used in the treatment of
mast cell tumors in animals, specifically dogs.
[1][2] Since its introduction in November 2008 it has been distributed under the commercial name
Masivet. It has been available in Europe since the second part of 2009. In the USA it is distributed under the name
Kinavet and has been available for veterinaries since 2011.
Masitinib is being studied for several human conditions including cancers. It is used in Europe to fight
orphan diseases.
[3]
Mechanism of action
Masitinib inhibits the
receptor tyrosine kinase c-Kit which is displayed by various types of tumour.
[2] It also inhibits the
platelet derived growth factor receptor (PDGFR) and
fibroblast growth factor receptor (FGFR).

……………………..
http://www.google.com/patents/US7423055
Compound Synthesis
General: All chemicals used were commercial reagent grade products.
Dimethylformamide (DMF), methanol (MeOH) were of anhydrous commercial
grade and were used without further purification. Dichloromethane and
tetrahydrofuran (THF) were freshly distilled under a stream of argon
before use. The progress of the reactions was monitored by thin layer
chromatography using precoated silica gel 60F 254, Fluka TLC plates,
which were visualized under UV light. Multiplicities in
1H
NMR spectra are indicated as singlet (s), broad singlet (br s), doublet
(d), triplet (t), quadruplet (q), and multiplet (m) and the NMR spectrum
were realized on a 300 MHz Bruker spectrometer.
3-Bromoacetyl-pyridine, HBr Salt
Dibromine (17.2 g, 108 mmol) was added dropwise to a cold (0° C.)
solution of 3-acetyl-pyridine (12 g, 99 mmol) in acetic acid containing
33% of HBr (165 mL) under vigourous stirring. The vigorously stirred
mixture was warmed to 40° C. for 2 h and then to 75° C. After 2 h at 75°
C., the mixture was cooled and diluted with ether (400 mL) to
precipitate the product, which was recovered by filtration and washed
with ether and acetone to give white crystals (100%). This material may
be recrystallised from methanol and ether.
IR (neat): 3108, 2047, 2982, 2559, 1709, 1603, 1221, 1035, 798 cm
−1—
−1H NMR (DMSO-d
6) δ=5.09 (s, 2H, CH
2Br); 7.88 (m, 1H, pyridyl-H); 8.63 (m, 1H, pyridyl-H); 8.96 (m, 1H, pyridyl-H); 9.29 (m, 1H, pyridyl-H).
Methyl-[4-(1-N-methyl-piperazino)-methyl]-benzoate
To methyl-4-formyl benzoate (4.92 g, 30 mmol) and N-methyl-piperazine
(3.6 mL, 32 mmol) in acetonitrile (100 mL) was added dropwise 2.5 mL of
trifluoroacetic acid. The reaction mixture was stirred at room
temperature for 1 h. After slow addition of sodium cyanoborohydride (2
g, 32 mmol), the solution was left stirring overnight at room
temperature. Water (10 mL) was then added to the mixture, which was
further acidified with 1N HCl to pH=6-7. The acetonitrile was removed
under reduced pressure and the residual aqueous solution was extracted
with diethyl ether (4×30 mL). These extracts were discarded. The aqueous
phase was then basified (pH>12) by addition of 2.5N aqueous sodium
hydroxyde solution. The crude product was extracted with ethyl acetate
(4×30 mL). The combined organic layers were dried over MgSO
4 and
concentrated under reduced pressure to afford a slightly yellow oil
which became colorless after purification by Kugelrohr distillation
(190° C.) in 68% yield.
IR(neat): 3322, 2944, 2802, 1721, 1612, 1457, 1281, 1122, 1012—
1H NMR(CDCl
3) δ=2.27 (s, 3H, NCH
3); 2.44 (m, 8H, 2×NCH
2CH
2N); 3.53 (s, 2H, ArCH
2N); 3.88 (s, 3H, OCH
3); 7.40 (d, 2H, J=8.3 Hz, 2×ArH); 7.91 (d, 2H, J=8.3 Hz, 2×ArH)—
3C NMR (CDCl
3) δ=45.8 (NCH
3); 51.8 (OCH
3); 52.9 (2×CH
2N); 54.9 (2×CH
2N); 62.4 (ArCH
2N); 128.7 (2×ArC); 129.3 (2×ArC); 143.7 (ArC); 166.7 (ArCO
2CH
3)-MS CI (m/z) (%) 249 (M+1, 100%).
2-Methyl-5-tert-butoxycarbonylamino-aniline
A solution of di-tert-butyldicarbonate (70 g, 320 mmol) in methanol
(200 mL) was added over 2 h to a cold (−10° C.) solution of
2,4-diaminotoluene (30 g, 245 mmol) and triethylamine (30 mL) in
methanol (15 mL). The reaction was followed by thin layer chromatography
(hexane/ethyl acetate, 3:1) and stopped after 4 h by adding 50 mL of
water. The mixture was concentrated in vacuo and the residue was
dissolved in 500 mL of ethyl acetate. This organic phase was washed with
water (1×150 mL) and brine (2×150 mL), dried over MgSO
4, and
concentrated under reduced pressure. The resulting light brown solid
was washed with small amounts of diethyl ether to give off-white
crystals of 2-methyl-5-tert-butoxycarbonylamino-aniline in 67% yield.
IR (neat): 3359; 3246; 2970; 1719; 1609; 1557; 1173; 1050 cm
−1—
1H NMR (CDCl
3): δ=1.50 (s, 9H, tBu); 2.10 (s, 3H, ArCH
3); 3.61 (br s, 2H, NH
2); 6.36 (br s, 1H, NH); 6.51 (dd, 1H, J=7.9 Hz, 2.3 Hz, ArH); 6.92 (d, 1H, J=7.9 Hz, ArH); 6.95 (s, 1H, ArH)—
13C NMR (CDCl
3) δ=16.6 (ArCH
3); 28.3 (C(CH
3)
3); 80.0 (C(CH
3)
3); 105.2 (ArC); 108.6 (ArC); 116.9 (ArC); 130.4 (ArC—CH
3); 137.2 (ArC—NH); 145.0 (ArC—NH
2); 152.8 (COOtBu) MS ESI (m/z) (%): 223 (M+1), 167 (55, 100%).
N-(2-methyl-5-tert-butoxycarbonylamino)phenyl-thiourea
Benzoyl chloride (5.64 g, 80 mmol) was added dropwise to a
well-stirred solution of ammonium thiocyanate (3.54 g, 88 mmol) in
acetone (50 mL). The mixture was refluxed for 15 min, then, the
hydrobromide salt of 2-methyl-5-tert-butoxycarbonylamino-aniline (8.4 g,
80 mmol) was added slowly portionswise. After 1 h, the reaction mixture
was poured into ice-water (350 mL) and the bright yellow precipitate
was isolated by filtration. This crude solid was then refluxed for 45
min in 70 mL of 2.5 N sodium hydroxide solution. The mixture was cooled
down and basified with ammonium hydroxide. The precipitate of crude
thiourea was recovered by filtration and dissolved in 150 mL of ethyl
acetate. The organic phase was washed with brine, dried over Na
2SO
4,
and concentrated under reduced pressure. The residue was purified by
column chromatography (hexane/ethyl acetate, 1:1) to afford 63% of
N-(2-methyl-5-tert-butoxycarbonylamino)phenyl-thiourea as a white solid.
IR (neat): 3437, 3292, 3175, 2983, 1724, 1616, 1522, 1161, 1053 cm
−1— 1H NMR (DMSO-d
6) δ=1.46 (s, 9H, tBu); 2.10 (s, 3H, ArCH
3); 3.60 (br s, 2H, NH
2);
7.10 (d, 1H, J=8.29 Hz, ArH); 7.25 (d, 1H, J=2.23 Hz, ArH); 7.28 (d,
1H, J=2.63 Hz, ArH); 9.20 (s, 1H, ArNH); 9.31 (s, 1H, ArNH)—
13C NMR (DMSO-d
6) δ=25.1 (ArCH
3); 28.1 (C(CH
3)
3); 78.9 (C(CH
3)
3); 16.6 (ArC); 117.5 (ArC); 128.0 (ArC); 130.4 (ArC—CH
3);
136.5 (ArC—NH); 137.9 (ArC—NH); 152.7 (COOtBu); 181.4 (C═S)—MS CI(m/z):
282 (M+1, 100%); 248 (33); 226 (55); 182 (99); 148 (133); 93 (188).
2-(2-methyl-5-tert-butoxycarbonylamino)phenyl-4-(3-pyridyl)-thiazole
A mixture of 3-bromoacetyl-pyridine, HBr salt (0.81 g, 2.85 mmol),
N-(2-methyl-5-tert-butoxycarbonylamino)phenyl-thiourea (0.8 g, 2.85
mmol) and KHCO
3 (˜0.4 g) in ethanol (40 mL) was heated at 75° C. for 20 h. The mixture was cooled, filtered (removal of KHCO
3) and evaporated under reduced pressure. The residue was dissolved in CHCl
3 (40 mL) and washed with saturated aqueous sodium hydrogen carbonate solution and with water. The organic layer was dried over Na
2SO
4 and
concentrated. Colum chromatographic purification of the residue
(hexane/ethyl acetate, 1:1) gave the desired thiazole in 70% yield as an
orange solid
IR(neat): 3380, 2985, 2942, 1748, 1447, 1374, 1239, 1047, 938—
1H NMR (CDCl
3) δ=1.53 (s, 9H, tBu); 2.28 (s, 3H, ArCH
3);
6.65 (s, 1H, thiazole-H); 6.89 (s, 1H); 6.99 (dd, 1H, J=8.3 Hz, 2.3
Hz); 7.12 (d, 2H, J=8.3 Hz); 7.35 (dd, 1H, J=2.6 Hz, 4.9 Hz); 8.03 (s,
1H); 8.19 (dt, 1H, J=1.9 Hz, 7.9 Hz); 8.54 (br s, 1H, NH); 9.09 (s, 1H,
NH)—
13C NMR (CDCl
3) δ=18.02 (ArCH
3); 29.2 (C(CH
3)
3); 81.3 (C(CH
3)
3);
104.2 (thiazole-C); 111.6; 115.2; 123.9; 124.3; 131.4; 132.1; 134.4;
139.5; 148.2; 149.1; 149.3; 153.6; 167.3 (C═O)—MS Cl (m/z) (%): 383
(M+1, 100%); 339 (43); 327 (55); 309 (73); 283 (99); 71 (311).
2-(2-methyl-5-amino)phenyl-4-(3-pyridyl)-thiazole
2-(2-methyl-5-tert-butoxycarbonylamino)phenyl-4-(3-pyridyl)-thiazole (0.40 g, 1.2 mmol) was dissolved in 10 mL of 20% TFA/CH
2Cl
2.
The solution was stirred at rool temperature for 2 h, then it was
evaporated under reduced pressure. The residue was dissolved in ethyl
acetate. The organic layer was washed with aqueous 1N sodium hydroxide
solution, dried over MgSO
4, and concentrated to afford
2-(2-methyl-5-amino)phenyl-4-(3-pyridyl)-thiazole as a yellow-orange
solid in 95% yield. This crude product was used directly in the next
step.
A 2M solution of trimethyl aluminium in toluene (2.75 mL) was added
dropwise to a cold (0° C.) solution of
2-(2-methyl-5-amino)phenyl-4-(3-pyridyl)-thiazole (0.42 g, 1.5 mmol) in
anhydrous dichloromethane (10 mL) under argon atmosphere. The mixture
was warmed to room temperature and stirred at room temperature for 30
min. A solution of methyl-4-(1-N-methyl-piperazino)-methyl benzoate
(0.45 g, 1.8 mmol) in anhydrous dichloromethane (1 mL) and added slowly,
and the resulting mixture was heated at reflux for 5 h. The mixture was
cooled to 0° C. and quenched by dropwise addition of a 4N aqueous
sodium hydroxide solution (3 mL). The mixture was extracted with
dichloromethane (3×20 mL). The combined organic layers were washed with
brine (3×20 mL) and dried over anhydrous MgSO
4.
(2-(2-methyl-5-amino)phenyl-4-(3-pyridyl)-thiazole) is obtained in 72%
after purification by column chromatography (dichloromethane/methanol,
3:1)
IR (neat): 3318, 2926, 1647, 1610, 1535, 1492, 1282, 1207, 1160, 1011, 843—
1H NMR (CDCl3) δ=2.31 (br s, 6H, ArCH3+NCH3); 2.50 (br s, 8H, 2×NCH2CH2N); 3.56 (s, 2H, ArCH2N);
6.89 (s, 1H, thiazoleH); 7.21-7.38 (m, 4H); 7.45 (m, 2H); 7.85 (d, 2H,
J=8.3 Hz); 8.03 (s, 1H); 8.13 (s, 1H); 8.27 (s, 1H); 8.52 (br s, 1H);
9.09 (s, 1H, NH)—
13C NMR (CDCl3) δ 17.8 (ArCH3); 46.2 (NCH3); 53.3 (NCH2); 55.3 (NCH2); 62.8 (ArCH2N);
99.9 (thiazole-C); 112.5; 123.9; 125.2; 127.5; 129.6; 131.6; 133.7;
134.0; 137.6; 139.3; 142.9; 148.8; 149.1; 166.2 (C═O); 166.7
(thiazoleC-NH)—
MS CI (m/z) (%): 499 (M+H, 100%); 455 (43); 430 (68); 401 (97); 374 (124); 309 (189); 283 (215); 235 (263); 121 (377); 99 (399).
………………………
http://www.google.com/patents/WO2012136732A1?cl=en
In a preferred embodiment of the above-depicted treatment, the active
ingredient masitinib is administered in the form of masitinib mesilate;
which is the orally bioavailable mesylate salt of masitinib – CAS
1048007-93-7 (MsOH); C28H30N6OS.CH3SO3H; MW 594.76:
http://www.google.com/patents/WO2004014903A1?cl=en
003 : 4-(4-Methyl-piperazin-l-ylmethyl)-N-[3-(4-pyridin-3-yl-thiazol-2-ylamino)- phenyl] -benzamide
4-(4-Methyl-piperazin-l-yl)-N-[4-methyl-3-(4-pyridin-3-yl-thiazol-2-ylmethyl)- phenyl] -benzamide
beige brown powder mp : 128-130°C
1H RMN (DMSO-d6) δ = 2.15 (s,
3H) ; 2.18 (s, 3H) ; 2.35-2.41 (m, 4H) ; 3.18-3.3.24 (m, 4H) ; 6.94 (d,
J = 8.9 Hz, 2H) ; 7.09 (d, J = 8.4 Hz, IH) ; 7.28-7.38 (m, 3H) ; 7.81
(d, J = 8.9 Hz, 2H) ; 8.20-8.25 (m, IH) ; 8.40 (dd, J = 1.6 Hz, J = 4.7 ,
IH) ; 8.48 (d, J = 1.9 Hz, IH) ; 9.07 (d, J = 1.5 Hz, IH) ; 9.35 (s,
IH) ; 9.84 (s, IH)

……………
http://www.google.com/patents/WO2008098949A2?cl=en
EXAMPLE 4 N- [4-Methyl-3 -(4-pyridin-3 -yl-thiazol-2-ylamino)-phenyl] -benzamide derivatives
Method A In a reactor and under low nitrogen pressure, add
4-Methyl-N3-(4-pyridin-3-yl-thiazol- 2-yl)-benzene-l,3-diamine (95 g,
336.45 mmol), dichloromethane (2 L). To this suspension cooled to
temperature of 5°C was added dropwise 2M/n-hexane solution of
trimethylaluminium (588 mL). The reaction mixture was brought
progressively to 15°C, and maintained for 2 h under stirring.
4-(4-Methyl-piperazin-l-ylmethyl)-benzoic acid methyl ester (100 g,
402.71 mmol) in dichloromethane (200 mL) was added for 10 minutes. After
1 h stirring at room temperature, the reaction mixture was heated to
reflux for 20 h and cooled to room temperature. This solution was
transferred dropwise via a cannula to a reactor containing 2N NaOH (2.1
L) cooled to 5°C. After stirring for 3 h at room temperature, the
precipitate was filtered through Celite. The solution was extracted with
dichloromethane and the organic layer was washed with water and
saturated sodium chloride solution, dried over MgSO
4 and concentrated under vacuum. The brown solid obtained was recrystallized from /-Pr
2O to give 130.7 g (78%) of a beige powder.
Method B Preparation of the acid chloride
To a mixture of 4-(4-Methyl-piperazin-l-ylmethyl)-benzoic acid
dihydrochloride (1.0 eq), dichloromethane (7 vol) and triethylamine
(2.15 eq), thionyl chloride (1.2 eq) was added at 18-28°C . The reaction
mixture was stirred at 28-32°C for 1 hour. Coupling of acid chloride
with amino thiazole To a chilled (0-5
0C) suspension of
4-Methyl-N3-(4-pyridin-3-yl-thiazol-2-yl)-benzene- 1,3-diamine (0.8 eq)
and thiethylamine (2.2 eq) in dichloromethane (3 vol), the acid chloride
solution (prepared above) was maintaining the temperature below 5°C.
The reaction mixture was warmed to 25-30
0C and stirred at the
same temperature for 1O h. Methanol (2 vol) and water (5 vol) were
added to the reaction mixture and stirred. After separating the layers,
methanol (2 vol), dihloromethane (5 vol) and sodium hydroxide solution
(aqueous, 10%, till pH was 9.5-10.0) were added to the aqueous layer and
stirred for 10 minutes. The layers were separated. The organic layer
was a washed with water and saturated sodium chloride solution. The
organic layer was concentrated and ethanol (2 vol) was added and
stirred. The mixture was concentrated. Ethanol was added to the residue
and stirred. The product was filtered and dried at 50-55
0C in a vaccum tray drier. Yield = 65-75%.
Method C
To a solution of
4-methyl-N3-(4-pyridin-3-yl-thiazol-2-yl)-benzene-l,3-diamine (1.0 eq)
in DMF (20 vol) were added successively triethylamine (5 eq),
2-chloro-l- methylpyridinium iodide (2 eq) and
4-(4-methyl-piperazin-l-ylmethyl)-benzoic acid (2 eq). The reaction
mixture was stirred for 7 h at room temperature. Then, the mixture was
diluted in diethyl ether and washed with water and saturated aqueous
NaHCO3, dried over Na2SO4 and concentrated. The crude product was
purified by column chromatography using an elution of 100% EtOAc to give
a yellow solid.
Yield = 51%.
1H NMR (CDCl
3) : δ = 9.09 (IH, s, NH); 8.52 (IH, br s); 8.27 (IH, s); 8.13 (IH, s);
8.03 (IH, s); 7.85 (2H, d, J= 8.3Hz); 7.45 (2H, m); 7.21-7.38 (4H, m); 6.89 (IH, s);
3.56 (2H, s); 2.50 (8H, br s); 2.31 (6H, br s).
MS (CI) m/z = 499 (M+H)
+.
An additional aspect of the present invention relates to a particular
polymorph of the methanesulfonic acid salt of
N-[4-Methyl-3-(4-pyridin-3-yl-thiazol-2-ylamino)-phenyl]- benzamide of
formula (IX).
(VI)
Hereinafter is described the polymorph form of (IX) which has the
most advantageous properties concerning processability, storage and
formulation. For example, this form remains, dry at 80% relative
humidity and thermodynamically stable at temperatures below 200
0C.
The polymorph of this form is characterized by an X-ray diffraction
pattern illustrated in FIG.I, comprising characteristic peaks
approximately 7.269, 9.120, 11.038, 13.704, 14.481, 15.483, 15.870,
16.718, 17.087, 17.473, 18.224, 19.248, 19.441, 19.940, 20.441, 21.469,
21.750, 22.111, 23.319, 23.763, 24.120, 24.681, 25.754, 26.777, 28.975,
29.609, 30.073 degrees θ, and is also characterized by differential
scanning calorimetry (DSC) illustrated in FIG.II, which exhibit a single
maximum value at approximately 237.49 ± 0.3
0C. X-ray
diffraction pattern is measured using a Bruker AXS (D8 advance).
Differential scanning calorimetry (DSC) is measured using a Perking
Elmer Precisely (Diamond DSC).
This polymorph form can be obtained by treatement of
4-(4-Methyl-piperazin-l-
ylmethyl)-N-[4-methyl-3-(4-pyridin-3-yl-thiazol-2-ylamino)-phenyl]-benzamide
with 1.0 to 1.2 equivalent of methanesulfonic acid, at a suitable
temperature, preferably between 20-80
0C.
The reaction is performed in a suitable solvent especially polar
solvent such as methanol or ethanol, or ketone such as acetone, or ether
such as diethylether or dioxane, or a mixture therof. This invention is
explained in example given below which is provided by way of
illustration only and therefore should not be construed to limit the
scope of the invention. Preparation of the above-mentioned polymorph
form of 4-(4-Methyl-piperazin-l- ylmethyl)-N- [4-methyl-3 -(4-pyridin-3
-yl-thiazol-2-ylamino)-phenyl] -benzamide methanesulfonate .
4-(4-Methyl-piperazin- 1 -ylmethyl)-N- [4-methyl-3 -(4-pyridin-3
-yl-thiazol-2-ylamino) phenyl] -benzamide (1.0 eq) was dissolved in
ethanol (4.5 vol) at 65-70
0C. Methanesulfonic acid (1.0 eq) was added slowly at the same temperature. The mixture was cooled to 25-30
0C and maintained for 6 h. The product was filtered and dried in a vacuum tray drier at 55-60
0C. Yield = 85-90%. Starting melting point Smp = 236°C.
NMR PREDICT
CAS NO. 1048007-93-7, methanesulfonic
acid,4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide
H-NMR spectral analysis
![methanesulfonic acid,4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide NMR spectra analysis, Chemical CAS NO. 1048007-93-7 NMR spectral analysis, methanesulfonic acid,4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide H-NMR spectrum](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_tMJ36FqNHAp0cOL3Iu_33T-Zn2l8F_0_tjHFjuHOUY2WtZChgLWDEDLf4KNH506OostM1vmoe7rQzVM4hY_4uQijMybt2OJa7Vw9MKjh3DP0ISHil6NytSrrALNmhpDT6e41NtHXUchAdnbA=s0-d)
![methanesulfonic acid,4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide NMR spectra analysis, Chemical CAS NO. 1048007-93-7 NMR spectral analysis, methanesulfonic acid,4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide C-NMR spectrum](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_u8CD4nSYwcUaASUtxgbduTkOi0nB-H434CsEe0dlqtZlEIdM8hn8YKDfkgTdYTSf9sCyMUM5PE5Q-NlVy3O4-nqBWbQCFR6n30S_PpPFhy1L0Ryt_T2ccIAlRhjrcbG3RngMfzwiQjQ3X_iTo=s0-d)
CAS NO. 1048007-93-7, methanesulfonic acid,
4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-yl-1,3-thiazol-2-yl)amino]phenyl]benzamide
C-NMR spectral analysisPREDICT
References
- Hahn,
K.A.; Oglivie, G.; Rusk, T.; Devauchelle, P.; Leblanc, A.; Legendre,
A.; Powers, B.; Leventhal, P.S.; Kinet, J.-P.; Palmerini, F.; Dubreuil,
P.; Moussy, A.; Hermine, O. (2008). “Masitinib is Safe and Effective for
the Treatment of Canine Mast Cell Tumors”. Journal of Veterinary Internal Medicine 22 (6): 1301–1309. doi:10.1111/j.1939-1676.2008.0190.x. ISSN 0891-6640.
- Information about Masivet at the European pharmacy agency website
- Orphan designation for Masitinib at the European pharmacy agency website
| WO2004014903A1 |
Jul 31, 2003 |
Feb 19, 2004 |
Ab Science |
2-(3-aminoaryl)amino-4-aryl-thiazoles and their use as c-kit inhibitors |
| WO2008098949A2 |
Feb 13, 2008 |
Aug 21, 2008 |
Ab Science |
Process for the synthesis of 2-aminothiazole compounds as kinase inhibitors |
| EP1525200B1 |
Jul 31, 2003 |
Oct 10, 2007 |
AB Science |
2-(3-aminoaryl)amino-4-aryl-thiazoles and their use as c-kit inhibitors |
| US7423055 |
Aug 1, 2003 |
Sep 9, 2008 |
Ab Science |
2-(3-Aminoaryl)amino-4-aryl-thiazoles for the treatment of diseases |
| US20080207572 * |
Jul 13, 2006 |
Aug 28, 2008 |
Ab Science |
Use of Dual C-Kit/Fgfr3 Inhibitors for Treating Multiple Myeloma |
| Patent |
Submitted |
Granted |
| 2-(3-Aminoaryl)amino-4-aryl-thiazoles for the treatment of diseases [US7423055] |
2004-06-10 |
2008-09-09 |
| 2-(3-aminoaryl)amino-4-aryl-thiazoles and their use as c-kit inhibitors [US2005239852] |
2005-10-27 |
|
| Use of C-Kit Inhibitors for Treating Fibrosis [US2007225293] |
2007-09-27 |
|
| Use of Mast Cells Inhibitors for Treating Patients Exposed to Chemical or Biological Weapons [US2007249628] |
2007-10-25 |
|
| Use of c-kit inhibitors for treating type II diabetes [US2007032521] |
2007-02-08 |
|
| Use of tyrosine kinase inhibitors for treating cerebral ischemia [US2007191267] |
2007-08-16 |
|
| Use of C-Kit Inhibitors for Treating Plasmodium Related Diseases [US2008004279] |
2008-01-03 |
|
| Tailored Treatment Suitable for Different Forms of Mastocytosis [US2008025916] |
2008-01-31 |
|
| 2-(3-AMINOARYL) AMINO-4-ARYL-THIAZOLES AND THEIR USE AS C-KIT INHIBITORS [US2008255141] |
2008-10-16 |
|
| Use Of C-Kit Inhibitors For Treating Inflammatory Muscle Disorders Including Myositis And Muscular Dystrophy [US2008146585] |
2008-06-19 |
| Patent |
Submitted |
Granted |
| Aminothiazole compounds as kinase inhibitors and methods of using the same [US8940894] |
2013-05-10 |
2015-01-27 |
| Aminothiazole compounds as kinase inhibitors and methods of using the same [US8492545] |
2012-03-08 |
2013-07-23 |
| Patent |
Submitted |
Granted |
| Use of Dual C-Kit/Fgfr3 Inhibitors for Treating Multiple Myeloma [US2008207572] |
2008-08-28 |
| PROCESS FOR THE SYNTHESIS OF 2-AMINOTHIAZOLE COMPOUNDS AS KINASE INHIBITORS [US8153792] |
2010-05-13 |
2012-04-10 |
| COMBINATION TREATMENT OF SOLID CANCERS WITH ANTIMETABOLITES AND TYROSINE KINASE INHIBITORS [US8227470] |
2010-04-15 |
2012-07-24 |
| Anti-IGF antibodies [US8580254] |
2008-06-19 |
2013-11-12 |
| COMBINATIONS FOR THE TREATMENT OF B-CELL PROLIFERATIVE DISORDERS [US2009047243] |
2008-07-17 |
2009-02-19 |
| TREATMENTS OF B-CELL PROLIFERATIVE DISORDERS [US2009053168] |
2008-07-17 |
2009-02-26 |
| Anti-IGF antibodies [US8318159] |
2009-12-11 |
2012-11-27 |
| SURFACE TOPOGRAPHIES FOR NON-TOXIC BIOADHESION CONTROL [US2010226943] |
2009-08-31 |
2010-09-09 |
| EGFR/NEDD9/TGF-BETA INTERACTOME AND METHODS OF USE THEREOF FOR THE
IDENTIFICATION OF AGENTS HAVING EFFICACY IN THE TREATMENT OF
HYPERPROLIFERATIVE DISORDERS [US2010239656] |
2010-05-10 |
2010-09-23 |
| ANTI CD37 ANTIBODIES [US2010189722] |
2008-08-08 |
2010-07-29 |
United States National Library of Medicine
Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, Borge L, Hajem B, Lermet A, Sippl W, et al.
Food and Drug Administration, 15 Dec 2010
European Medicines Agency, Nov 2008
Piette F, Belmin J, Vincent H, Schmidt N, Pariel S, Verny M, Marquis C, Mely J, Hugonot-Diener L, Kinet J-P, et al.
Tebib J, Mariette X, Bourgeois P, Flipo R-M, Gaudin P, Le Loët X, Gineste P, Guy L, Mansfield CD, Moussy A, et al.
Businesswire.com, 6 Sep 2011
ClinicalTrials.gov, 13 Sep 2011
ClinicalTrials.gov, 10 Oct 2011
Le Cesne A, Blay J-Y, Bui B N, Bouché O, Adenis A, Domont J, Cioffi A, Ray-Coquard I, Lassau N, Bonvalot S, et al.
Editors’ Pick
Vermersch P, Benrabah R, Schmidt N, Zéphir H, Clavelou P, Vongsouthi C, Dubreuil P, Moussy A, Hermine O
The Wall Street Journal, 4 Nov 2013
Kocic I, Kowianski P, Rusiecka I, Lietzau G, Mansfield C, Moussy A, Hermine O, Dubreuil P
TAJIKISTAN
en.wikipedia.org/wiki/Tajikistan
The territory that now constitutes Tajikistan was previously home to several ancient cultures, including the city of Sarazm of the Neolithic and the Bronze Age, …

.







The nature of Tajikistan. Nurek
Tajikistan. Pamiro-Alay.Zeravshan mountain range. Guzn village. Local people
Dushanbe, Tajikistan
Women carry water canisters near Gargara village, 110km south of Tajikistan’s capital, Dushanbe
Ancient Buddhist ruins, Ajina Teppa, Tajikistan
///////////
Share this: