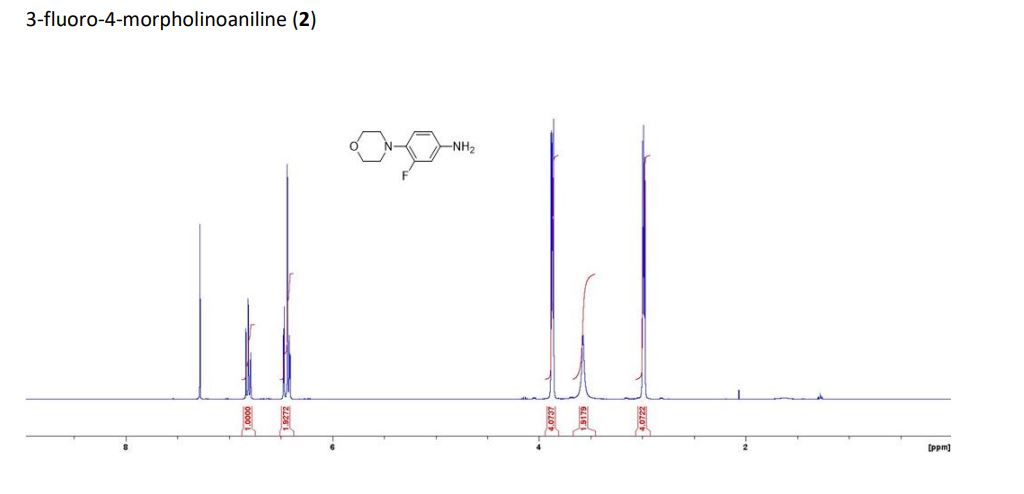
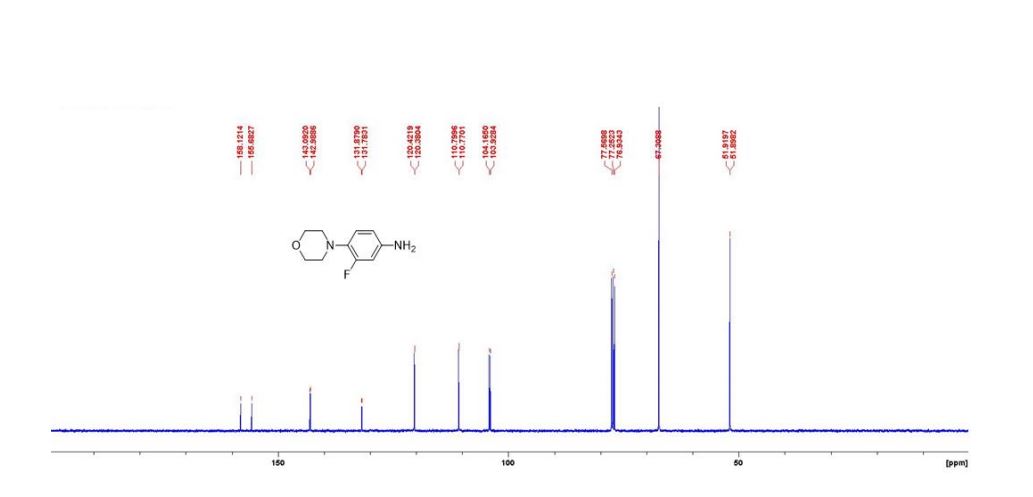
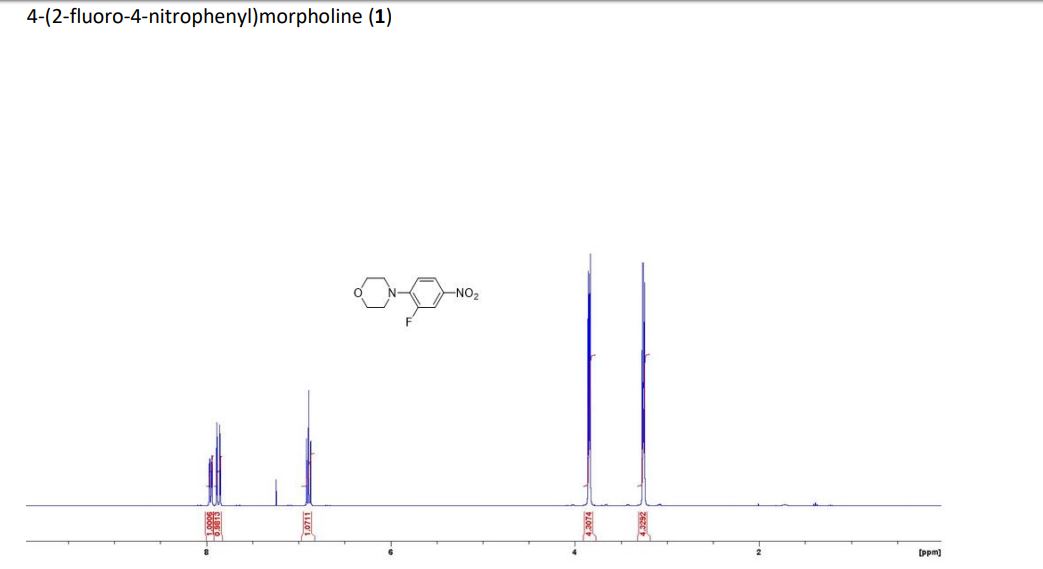
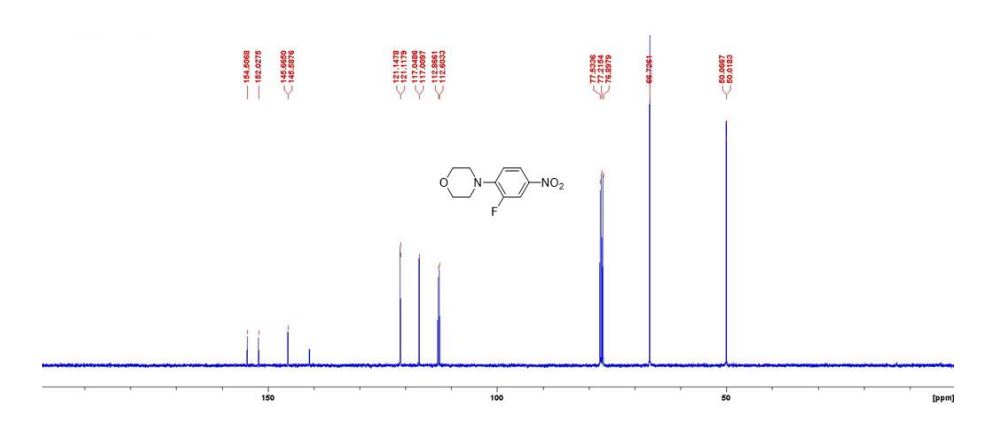
3-fluoro-4- morpholinoaniline
1H NMR (400MHz, CDCl3) 6.82 (m, 1H, ArH), 6.43 (m, 2H, 2xArH), 3.87 (m, 4H, 2xCH2O), 3.58 (brs, 2H, NH2), 2.99 (m, 4H, 2xCH2N). 13C NMR (100MHz, CDCl3) 156.9 (d J= 245.4Hz), 143.0 (d J=10.4Hz), 131.8 (d J=9.7Hz), 120.4 (d J=4.2Hz), 110.8 (d J=3.0Hz), 104.0 (d J=23.8Hz), 67.3, 51.9 (d J=2.1Hz). HRMS [M] Calcd for C10H13FN2O 196.1006, Found 196.1004.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00153
////////////////////