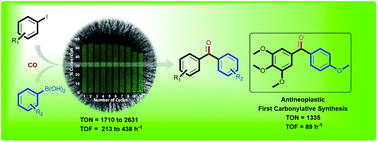
KCC-1 supported palladium nanoparticles as an efficient and sustainable nanocatalyst for carbonylative Suzuki-Miyaura cross-coupling
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02012G, Paper
DOI: 10.1039/C6GC02012G, Paper
Prashant Gautam, Mahak Dhiman, Vivek Polshettiwar, Bhalchandra M. Bhanage
This work reports a cost-effective and sustainable protocol for the carbonylative Suzuki-Miyaura cross-coupling reaction catalyzed by palladium nanoparticles (Pd NPs) supported on fibrous nanosilica (KCC-1) with very high turnover number.
This work reports a cost-effective and sustainable protocol for the carbonylative Suzuki-Miyaura cross-coupling reaction catalyzed by palladium nanoparticles (Pd NPs) supported on fibrous nanosilica (KCC-1) with very high turnover number.
This work reports a cost-effective and sustainable protocol for the carbonylative Suzuki–Miyaura cross-coupling reaction catalyzed by palladium nanoparticles (Pd NPs) supported on fibrous nanosilica (KCC-1). Under mild reaction conditions, the KCC-1-PEI/Pd catalytic system showed a turnover number (TON) 28-times and a turnover frequency (TOF) 51-times higher than the best supported Pd catalyst reported in the literature for the carbonylative cross-coupling between 4-iodoanisole and phenylboronic acid, as a test reaction. Also, the catalyst could be recycled up to ten times with a marginal loss in activity after the eighth cycle. The high activity of the catalyst can be attributed to the fibrous nature of the KCC-1 support and PEI functionalization provided the enhanced stability.
(4-methoxyphenyl)(phenyl)methanone (3b) 59.3 mg, yield 56%
1H NMR (500 MHz, CDCl3): δ 7.86 (d, J = 8.6 Hz, 2H), 7.78 (d, J = 7.6 Hz, 2H), 7.59 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 6.99 (d, J = 8.6 Hz, 2H), 3.92 (s, 3H).
13C{1H}NMR (125 MHz, CDCl3): δ 195.4, 163.1, 138.2, 132.4, 131.8, 130.0, 129.6, 128.1, 113.4, 55.4.
GCMS (EI, 70 eV): m/z (%): 212 (40), 135 (100), 105 (14), 77 (36).
KCC-1 supported palladium nanoparticles as an efficient and sustainable nanocatalyst for carbonylative Suzuki–Miyaura cross-coupling
*Corresponding authors
aDepartment of Chemistry, Institute of Chemical Technology, N.P. Marg, Matunga-400019, Mumbai, India
E-mail: bm.bhanage@ictmumbai.edu.in,bm.bhanage@gmail.com
E-mail: bm.bhanage@ictmumbai.edu.in,bm.bhanage@gmail.com
bNanocatalysis Laboratories (NanoCat), Department of Chemical Sciences, Tata Institute of Fundamental Research (TIFR), Homi Bhabha Road, Colaba, Mumbai, India
E-mail: vivekpol@tifr.res.in
E-mail: vivekpol@tifr.res.in
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC02012G

\\\\\\\\\\KCC-1 supported, palladium nanoparticles, sustainable nanocatalyst, carbonylative Suzuki-Miyaura cross-coupling, Prashant Gautam, Mahak Dhiman, Vivek Polshettiwar, Bhalchandra M. Bhanage