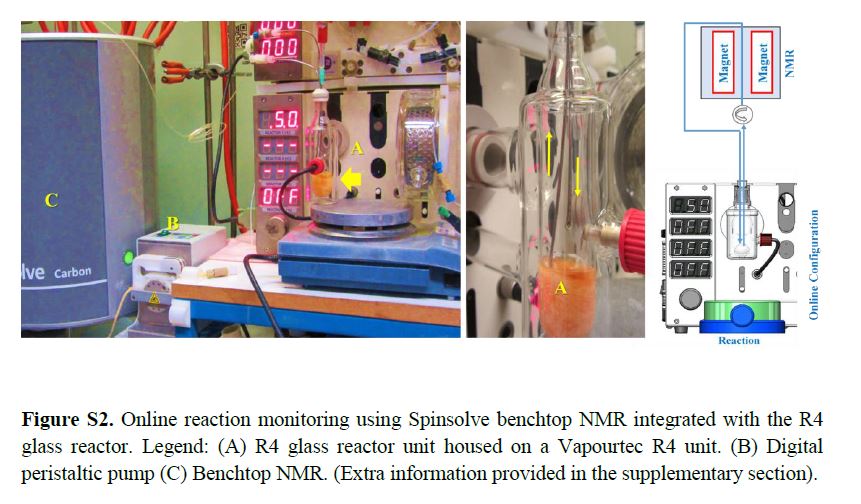
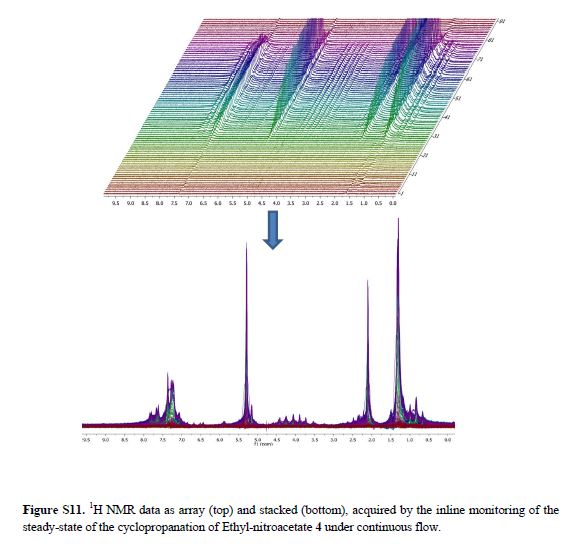
Real-time NMR spectroscopy has proven to be a rapid and an effective monitoring tool to study the hypervalent iodine(III) mediated cyclopropanation. With the ever increasing number of new synthetic methods for carbon–carbon bond formation, the NMR in situ monitoring of reactions is becoming a highly desirable enabling method. In this study, we have demonstrated the versatility of benchtop NMR using inline and online real-time monitoring methods to access mutually complementary information for process understanding, and we developed new approaches for real-time monitoring addressing challenges associated with better integration into continuous processes.
Continuous Processing and Efficient in Situ Reaction Monitoring of a Hypervalent Iodine(III) Mediated Cyclopropanation Using Benchtop NMR Spectroscopy
† Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, U.K.
‡Magritek GmbH, Gebäude VO (Building VO), Triwo Technopark Aachen, Philipsstrasse 8, 52068 Aachen, Germany
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00177
*Email: batoolahmedomer@gmail.com.
Steven V. Ley received his PhD from Loughborough University in 1972, after which he carried out post-doctoral research with Professor Leo Paquette at Ohio State University, followed by Professor Derek Barton at Imperial College London. In 1975, he joined that Department as a lecturer and became Head of Department in 1989. In 1992, he moved to the 1702 BP Chair of Organic Chemistry at the University of Cambridge and became a Fellow of Trinity College. He was elected to the Royal Society in 1990 and was President of the Royal Society of Chemistry (RSC) 2000-02. Steve has been the recipient of many prizes and awards including the Yamada-Koga Prize, Nagoya Gold Medal, ACS Award for Creative Work in Synthetic Organic Chemistry and the Paul Karrer Medal.
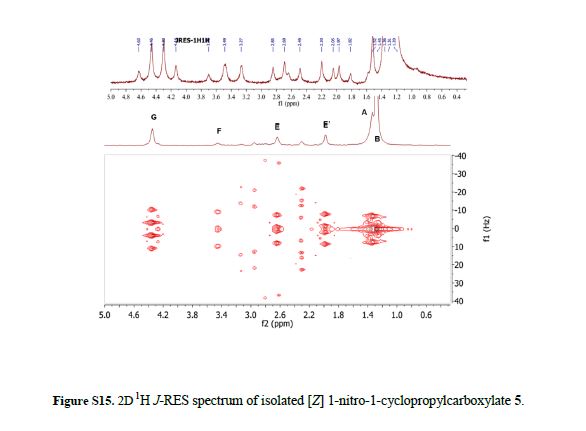
Ethyl 2-(4-tert-butylphenyl)-1-nitrocyclopropanecarboxylate (5):
[E]-isomer: 1H NMR (600 MHz, CDCl3): δ 0.80-0.85 (t, J = 7.1 Hz, 3H), 1.29 (s, 9H), 2.16-2.21 (dd, J = 10.7, 6.6 Hz, 1H), 2.41-2.46 (dd, J = 9.1, 6.6 Hz, 1H), 3.72-3.77 (m, 1H), 3.88-4.04 (m, 2H), 7.12-7.15 (d, J = 8.3 Hz, 2H), 7.30-7.37 (d, J = 8.4 Hz, 2H).
13C NMR (150 MHz, CDCl3) δ 161.96, 151.38, 128.96, 128.15, 125.37, 71.71, 62.37, 34.54, 33.91, 31.21, 20.73, 13.35.
HRMS (ESI) Calcd. for C16H21NO4 ([M+H]+): 292.15, Found 292.15:
[Z]-isomer: 1H NMR (600 MHz, CDCl3): δ 1.30 (s, 9H), 1.34-1.37 (t, J = 7.1 Hz, 3H), 2.00-2.04 (dd, J = 9.9, 6.9 Hz, 1H), 2.64-2.68 (dd, J = 9.2, 6.9 Hz, 1H), 3.43-3.48 (t, J = 9.6 Hz, 1H), 4.31-4.41 (m, 2H), 7.14-7.17 (d, J = 8.3 Hz, 2H), 7.32-7.36 (d, J = 8.4 Hz, 2H).
13C NMR (150 MHz, CDCl3) δ 165.40, 151.56, 128.33, 127.99, 125.63, 72.63, 63.14, 34.55, 33.48, 31.22, 20.08, 13.98.
Zhu, S.; Perman, J. A.; Zhang, X. P. Angew. Chem. Int. Ed. 2008, 47, 8460-8463.
ORGANIC CHEMISTRY RESEARCH GROUP

/////////