(5 R*,1′R*)-5-[3,5-Di-tert-butyl-4-hydroxyphenyl)hydroxymethyl]-2-ethyl-1,2-isothiazolidine-1,1-dioxide 12a (threo) and (5S*,1′R*)-5-[3,5-Di-tert-butyl-4-hydroxyphenyl)hydroxymethyl]-2-ethyl-1,2-isothiazolidine-1,1-dioxide 12b (erythro)
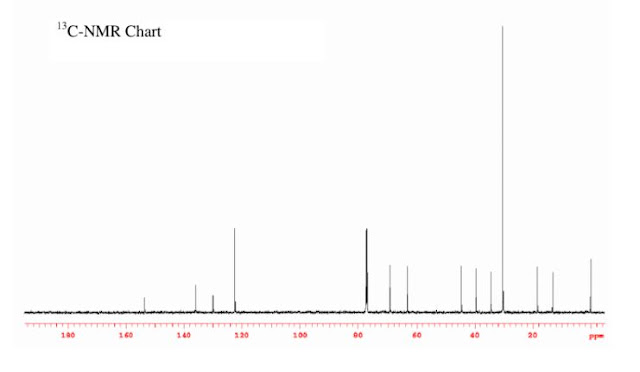
Compound 12a: mp 160−162 °C. 1H NMR (500 MHz, (CDCl3 δ) 1.25 (t, J = 7.3 Hz, 3H), 1.43 (s, 18H), 1.84 (m, 1H), 1.94 (m, 1H), 3.03 (ddd,J = 9.1, 8.2, 7.2 Hz, 1H), 3.08 (dq, J = 13.4, 7.2 Hz, 1H), 3.18 (ddd, J = 9.1, 8.0, 3.5 Hz, 1H), 3.23 (dq, J = 13.4, 7.2 Hz, 1H), 3.53 (m, 1H), 4.84 (d, J = 9.7 Hz 1H), 5.27 (s, 1H), 7.16 (s, 2H). 13C NMR (150 MHz, (CDCl3 δ) 13.18, 23.09, 30.24, 34.41, 39.42, 43.92, 63.67, 74.64, 123.58, 129.99, 136.38, 154.22. Elemental analysis: Calcd for C20H33O4NS: C; 62.63, H; 8.67, N; 3.65, S; 8.36, Found: C; 62.58, H; 8.62, N; 3.66, S; 8.32. Compound 12b: mp 78−96 °C. 1H NMR (500 MHz, (CDCl3 δ) 1.25 (t, J = 7.3 Hz, 3H), 1.44 (s, 18H), 2.08 (m, 1H), 2.60 (dq, J = 13.0, 8.5 Hz, 1H), 3.07 (m, 1H), 3.08 (m, 1H), 3.21 (dq, J = 13.3, 7.2 Hz, 1H), 3.28 (m, 1H), 3.31 (d, J = 2.6 Hz, 1H), 3.38 (td, J = 8.5, 2.1 Hz 1H), 5.21 (s, 1H), 5.40 (brs, 1H), 7.15 (s, 2H). 13C NMR (150 MHz, (CDCl3 δ) 13.18, 18.30, 30.28, 34.43, 39.46, 44.56, 63.21, 69.19, 122.41, 129.94, 136.14, 153.54. Elemental analysis: Calcd for C20H33O4NS: C; 62.63, H; 8.67, N; 3.65, S; 8.36, Found. C; 62.19, H; 8.63, N; 3.62, S; 8.10.
PAPER
Development of One-Pot Synthesis of New Antiarthritic Drug Candidate S-2474 with High E-Selectivity
Chemical Development Department, CMC Development Laboratories, Shionogi & Co., Ltd., 1-3, Kuise Terajima 2-chome, Amagasaki, Hyogo 660-0813, Japan, and Shionogi Research Laboratories, Shionogi & Co., Ltd., 12-4, Sagisu 5-chome, Fukushima-ku, Osaka 553-0002, Japan
Org. Process Res. Dev., 2008, 12 (3), pp 442–446
DOI: 10.1021/op800008w
* To whom correspondence should be addressed. Telephone: +81-6-6401-8198 . Fax: +81-6-6401-1371. E-mail:takemasa.hida@shionogi.co.jp., †
Chemical Development Department, CMC Development Laboratories.
, ‡Shionogi Research Laboratories.
A one-pot synthesis of S-2474 was developed to overcome the problems of a large number of steps, low stereoselectivity, low yield, a large amount of waste, and severe reaction conditions. Aldol-type condensation of 3,5-di-tert-butyl-4-hydroxybenzaldehyde and N-ethyl-γ-sultam was carried out with LDA and then quenched with water. Dehydration proceeded under basic conditions, providing S-2474 directly as a single isomer on the benzylidene double bond. The reaction mechanism appears to involve a quinone methide intermediate. Environmental assessment of the development of this compound is also discussed in this paper.
?///////New, Antiarthritic , Drug Candidate, S-2474, Shionogi Research Laboratories, cyclooxygenase-2, (COX-2), 5-lipoxygenase , (5-LO),






No comments:
Post a Comment