Multicomponent-Multicatalyst Reactions (MC)2R: Efficient Dibenzazepine SynthesisJennifer Tsoung, Jane Panteleev, Matthias Tesch, and Mark Lautens
A RhI/Pd0 catalyst system was applied to the multicomponent synthesis of aza-dibenzazepines from vinylpyridines, arylboronic acids, and amines in a domino process with no intermediate isolation or purification.
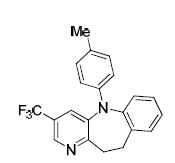
5-(p-tolyl)-3-(trifluoromethyl)-10,11-dihydro-5H-benzo[b]pyrido[2,3-f]azepine (4a)
1H NMR
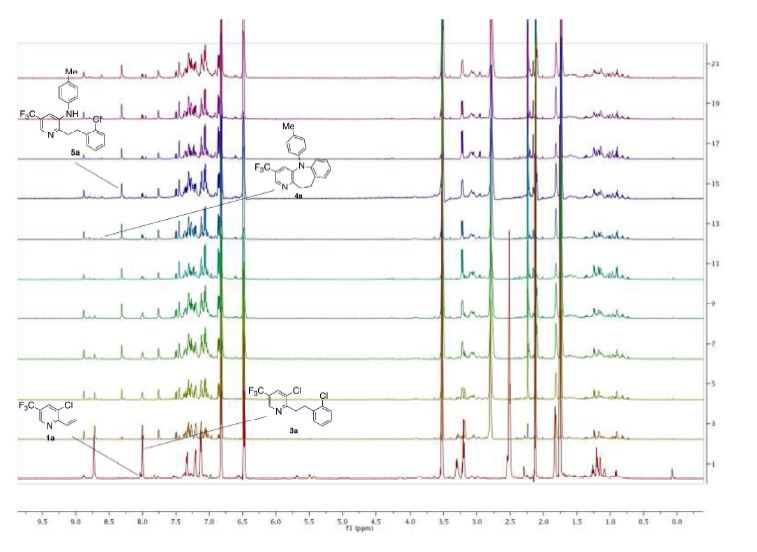
(400 MHz, CDCl3) δ 8.66 (d, J = 1.1 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.43 – 7.38 (m, 1H), 7.38 – 7.29
(m, 3H), 6.98 (d, J = 8.4 Hz, 2H), 6.57 – 6.51 (m, 2H), 3.33 – 3.21 (m, 2H), 3.09 – 2.99 (m, 2H), 2.26 (s,
3H);
(400 MHz, CDCl3) δ 8.66 (d, J = 1.1 Hz, 1H), 7.97 (d, J = 1.8 Hz, 1H), 7.43 – 7.38 (m, 1H), 7.38 – 7.29
(m, 3H), 6.98 (d, J = 8.4 Hz, 2H), 6.57 – 6.51 (m, 2H), 3.33 – 3.21 (m, 2H), 3.09 – 2.99 (m, 2H), 2.26 (s,
3H);
13C NMR (101 MHz, CDCl3) δ 161.7 (q, J = 1.3 Hz), 145.8, 143.6, 143.4 (q, J = 4.0 Hz), 139.7,
139.5, 134.9 (q, J = 3.5 Hz), 130.3, 130.0, 129.9, 128.9, 128.2, 127.7, 125.3 (q, J = 33.1 Hz), 123.4 (q, J =
272.5 Hz), 114.0 (2), 35.9, 29.0, 20.4;
139.5, 134.9 (q, J = 3.5 Hz), 130.3, 130.0, 129.9, 128.9, 128.2, 127.7, 125.3 (q, J = 33.1 Hz), 123.4 (q, J =
272.5 Hz), 114.0 (2), 35.9, 29.0, 20.4;
19F NMR (377 MHz, CDCl3) δ -62.0;
IR (NaCl, neat): 3063, 3028,
2926, 2862, 1616, 1506, 1489, 1456, 1435, 1429, 1410, 1339, 1319, 1296, 1267, 1240, 1207, 1165, 1128,
1086, 1036, 978, 947, 930, 910, 895, 808, 772, 756, 737, 721, 704, 687, 664, 646, 627 cm-1;
2926, 2862, 1616, 1506, 1489, 1456, 1435, 1429, 1410, 1339, 1319, 1296, 1267, 1240, 1207, 1165, 1128,
1086, 1036, 978, 947, 930, 910, 895, 808, 772, 756, 737, 721, 704, 687, 664, 646, 627 cm-1;
HRMS (ESI):
calcd for C21H18F3N2 (M+H)+: 355.1422; found. 355.1419.
calcd for C21H18F3N2 (M+H)+: 355.1422; found. 355.1419.

Jennifer Tsoung
PhD graduate, organic chemistry

Experience
PhD
University of Toronto
Research Intern
Kyoto University
Methodology project in asymmetric phase-transfer catalyzed alkylations.
Co-op student
Boehringer Ingelheim
On two hit-to-lead teams working to synthesize analogues of hit compounds for HIV research.
Publications
Diastereoselective Friedel−Crafts Alkylation of Hydronaphthalenes(Link)
The Journal of Organic Chemistry
September 27, 2011
An efficient and versatile synthesis of chiral tetralins has been developed using both inter- and intramolecular Friedel-Crafts alkylation as a key step. The readily available hydronaphthalene substrates were prepared via a highly enantioselective metal-catalyzed ring opening of meso-oxabicyclic alkenes followed by hydrogenation. A wide variety of complex tetracyclic compounds have been isolated...more
One-Pot Synthesis of Chiral Dihydrobenzofuran Framework via Rh/Pd Catlaysis
Organic Letters
October 12, 2012
A one-pot synthesis of the chiral dihydrobenzofuran framework is described. The method utilizes Rh-catalyzed asymmetric ring opening (ARO) and Pd-catalyzed C-O coupling to furnish the product in excellent enantioselectivity without isolation of intermediates. Systematic metal-ligand studies were carried out to investigate the compatibility of each catalytic system using product enantiopurity as an...more
Rh/Pd Catalysis with Chiral and Achiral Ligands: Domino Synthesis of Aza-Dihydrodibenzoxepines(Link)
Angew. Chem. Int. Ed
July 19, 2013
A game of dominoes: A synthetic route to aza-dihydrodibenzoxepines is described, through the combination of a Rh-catalyzed arylation and a Pd-catalyzed C-O coupling in a single pot. For the first time, the ability to incorporate a chiral and an achiral ligand in a two-component, two-metal transformation is achieved, giving the products in moderate to good yields, with excellent enantioselectivities.
Multicomponent-multicatalyst reactions (MC)(2)R: efficient dibenzazepine synthesis.
Organic Letters
January 13, 2014
A Rh(I)/Pd(0) catalyst system was applied to the multicomponent synthesis of aza-dibenzazepines from vinylpyridines, arylboronic acids, and amines in a domino process with no intermediate isolation or purification.
Formation of substituted oxa- and azarhodacyclobutanes.
Chemistry - A European Journal
December 6, 2013
The preparation of substituted oxa- and azarhodacyclobutanes is reported. After exchange of ethylene with a variety of unsymmetrically and symmetrically substituted alkenes, the corresponding rhodium-olefin complexes were oxidized with H2O2 and PhINTs (Ts=p-toluenesulfonyl) to yield the substituted oxa- and azarhodacyclobutanes, respectively. Oxarhodacyclobutanes could be prepared with excellent...more
Education
University of Toronto
Doctor of Philosophy (PhD), Organic Chemistry
Activities and Societies: Pueblo Science executive member, Let's Talk Science volunteer, International Chemistry Olympiad tutor
The University of British Columbia
Bachelor of Science (BSc), Chemistry
port moody secondary
High school

Mark Nitz presents Jennifer Tsoung certificate for her CTFP award

Women in Chemistry group, 2015
www.chem.utoronto.ca
July 2013

Mark Lautens , O.C.
University Professor
J. Bryan Jones Distinguished Professor
AstraZeneca Professor of Organic Chemistry
NSERC/Merck-Frosst Industrial Research Chair

Department of Chemistry
Davenport Chemical Laboratories
80 St. George St.
University of Toronto
Toronto, Ontario
M5S 3H6
Curriculum Vitae
Personal | ||
| Place and Date of Birth | Hamilton, Ontario, Canada | July 9, 1959 |
Education | ||
| Harvard University | NSERC PDF with D. A. Evans | 1985 - 1987 |
| University of Wisconsin-Madison | Ph.D. with B. M. Trost | 1985 |
| University of Guelph | B.Sc. - Distinction | 1981 |
Academic Positions | ||
| J. Bryan Jones Distinguished Professor | University of Toronto | 2013 - 2018 |
| University Professor | University of Toronto | 2012 - present |
| NSERC/Merck Frosst Industrial Research Chair | NSERC/Merck Frosst | 2003 - 2013 |
| AstraZeneca Professor of Organic Synthesis | University of Toronto | 1998 - present |
| Professor | University of Toronto | 1995 - 1998 |
| Associate Professor | University of Toronto | 1992 - 1995 |
| Assistant Professor | University of Toronto | 1987 - 1992 |
Awards & Honors | ||
| University of Toronto Alumni Faculty Award | University of Toronto | 2016 |
| CIC Catalysis Award | CSC | 2016 |
| Officer of the Order of Canada | Governor General | 2014 |
| Killam Research Fellowship | Canada Council for the Arts | 2013-2015 |
| CIC Medal | Chemical Institute of Canada | 2013 |
| Fellow of the Royal Society of UK | Royal Society of Chemistry | 2011 |
| Pedler Award | Royal Society of Chemistry | 2011 |
| Senior Scientist Award | Alexander von Humboldt Foundation Berlin, Aachen and Gottingen | 2009-2014 |
| Visiting Professor | University of Berlin | 2009 |
| Visiting Professor | Université de Marseilles | 2008 |
| ICIQ Summer School | ICIQ Tarragona, Spain | 2008 |
| Attilio Corbella Summer School Professor | Italian Chemical Society | 2007 |
| Arthur C. Cope Scholar Award | American Chemical Society | 2006 |
| Alfred Bader Award | Canadian Society for Chemistry | 2006 |
| R. U. Lemieux Award | Canadian Society for Chemistry | 2004 |
| Solvias Prize | Solvias AG | 2002 |
| Fellow of the Royal Society of Canada | Royal Society of Canada | 2001 |
Areas of Research Interest and Expertise | ||
| ||
///////Multicomponent, Multicatalyst Reactions, (MC)2R, Dibenzazepine Synthesis, Mark Lautens, University of Toronto , Toronto, Ontario, Jennifer Tsoung