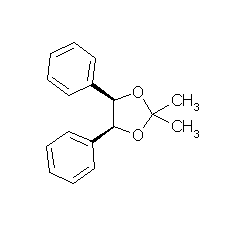
cis-2,2-Dimethyl-4,5-diphenyl-1,3-dioxolane
18699-77-9
1H NMR

| 1H-NMR: cis-2,2-Dimethyl-4,5-diphenyl-1,3-dioxolane | |||
| 300 MHz, CDCl3 | |||
| delta [ppm] | mult. | atoms | assignment |
| 1.62 | s | 3 H | CH3 |
| 1.84 | s | 3 H | CH3 |
| 4.84 | s | 1 H | CH-O (educt) |
| 5.53 | s | 2 H | CH-O (product) |
| 6.8-7.2 | m | 10 H | CH (arom., product) |
| 7.2-7.4 | m | 10 H | CH (arom., educt) |
| 7.26 | s | 1 H | CHCl3 |
13C-NMR |

| 13C-NMR: cis-2,2-Dimethyl-4,5-diphenyl-1,3-dioxolane | |||
| 300 MHz, CDCl3 | |||
| delta [ppm] | assignment | ||
| 24.5 | CH3 | ||
| 26.8 | CH3 | ||
| 81.5 | -OCH(R)-CH(R)-O- | ||
| 108.8 | O-C-O | ||
| 126.9-128.3 | CH arom. | ||
| 137.7 | C quart. arom. | ||
| 76.5-77.5 | CDCl3 | ||
IR

| IR: cis-2,2-Dimethyl-4,5-diphenyl-1,3-dioxolane | |||
| [KBr, T%, cm-1] | |||
| [cm-1] | assignment | ||
| 3095-3000 | arom. C-H valence | ||
| 2990-2880 | aliph. C-H valence | ||
| 1490, 1454 | arom. C=C valence | ||
 | + |  |
|  |
Synthesis of the acetonide of meso-1,2-diphenyl-1,2-ethanediol (2,2-dimethyl-4,5-diphenyl-1,3-dioxolane) |
| Reaction type: | reaction of the carbonyl group in ketones, acetalisation |
| Substance classes: | ketone, alcohol, acetal, protecting group, acid catalyst |
| Techniques: | heating under reflux, stirring with magnetic stir bar, filtering, evaporating with rotary evaporator, shaking out, extracting, recrystallizing, working with moisture exclusion, heating with oil bath |
Operating scheme |
 |
Classification Reaction types and substance classes reaction of the carbonyl group in ketones, acetalisation ketone, alcohol, acetal, protecting group, acid catalyst Work methods heating under reflux, stirring with magnetic stir bar, filtering, evaporating with rotary evaporator, shaking out, extracting, recrystallizing, working with moisture exclusion, heating with oil bath
Instruction (batch scale 5 mmol)
Equipment 100 mL three-neck flask, 50 mL round bottom flask, reflux condenser, protective gas supply, adapter with ground glass joint and hose coupling, heatable magnetic stirrer, magnetic stir bar, 250 mL beaker, 250 mL separating funnel, Hirsch funnel, suction flask, rotary evaporator, vacuum pump, oil bath Substances meso-1,2-diphenyl-1,2-ethanediol (mp 132-134 °C, 1.07 g (5.00 mmol) product from NOP 2004) iron(III)-chloride water free (mp 305 °C) 300 mg (1.85 mmol) acetone (bp 56 °C) dry 23.3 g (29.4 mL, 400 mmol) acetic acid ethyl ester (bp. 77 °C) 60 mL petroleum ether (40–60 °C) dry, for recrystallizing about 15 mL potassium carbonate about 1 g (for 50 mL of 2% aqueous solution) magnesium sulfate for drying about 1 g
Since FeCl3 is very hygroscopic, only a very short contact with air is allowed. The reaction apparatus consists of a dry 100 mL three-neck flask, rinsed with nitrogen and equipped with a magnetic stir bar and a reflux condenser, which is connected with a nitrogen piping. In the reaction flask 1.07 g (5.0 mmol) 1,2-diphenyl-1,2-ethanediol are dissolved in 23 g (30 mL, 400 mmol) dry acetone. 300 mg (1.85 mmol) of water free iron(III)-chloride are rapidly added, whilst the reaction mixture changes its colour to yellow-brown. It is heated under stirring for 20 minutes under reflux. Work up After cooling down to room temperature the reaction mixture is poured into a 250 mL beaker containing 50 mL of 2% potassium carbonate-solution. A brown fluffy precipitation is formed. The mixture is transferred into a 250 mL separating funnel and extracted three times with 20 mL acetic acid ethyl ester each. The organic phases are almost colourless. They are combined and again washed with 25 mL water and dried over water free magnesium sulfate. The mixture is filtered from the drying agent over a glass funnel with filter paper and the solvent is removed at a rotary evaporator. The light yellow oil remaining as crude product is dried for 30 minutes at about 40 °C bath temperature with a vacuum pump at 1-2 hPa. Crude yield: 1.05 g; mp 56 °C; HPLC-purity 83%
To the crude product are added 13 mL dry and pre-heated petroleum ether (40-60 °C) in a 50 mL round bottom flask and the mixture is stirred and heated for 5 minutes under reflux. Then the still hot solution is filtered off from the insoluble residue into a 50 mL round bottom flask and the solvent is evaporated at a rotary evaporator. The remaining light yellow oily product is dried with a vacuum pump at 1-2 hPa and stored in the cooler for crystallization. Yield: 0.918 g (3.61 mmol, 72%); mp 56-57 °C The product can be recrystallized from 1 mL dry petroleum ether (40-60 °C), however, it is still not free from educt yet. Yield: 0.52 g (2.05 mmol, 41%); mp 58-60 °C The residue (120 mg) which is insoluble in the hot petroleum ether consists predominantly of not reacted 1,2-diphenylethanediol.
Comments The product in solid state is stable under moisture exclusion at room temperature. A solution of the product in CDCl3 shows already after one day partly cleavage into the educts, probably due to traces of acid (detection with 1H NMR).
Substances required |
| Educts | Amount | Risk | Safety | ||||||
|---|---|---|---|---|---|---|---|---|---|
| meso-1,2-Diphenyl-1,2-ethanediol | 1.07 g | ||||||||
| Acetone |
| 23.3 g | H225 H319 H336 EUH066 | P210 P261 P305 + 351 + 338 | |||||
| Catalyst | Amount | Risk | Safety | ||||||
| Ferric chloride |
| 0.300 g | H290 H302 H315 H318 H411 | P273 P280 P305 + 351 + 338 | |||||
| Solvents | Amount | Risk | Safety | ||||||
| Acetic acid ethyl ester |
| 60 mL | H225 H319 H336 EUH066 | P210 P261 P305 + 351 + 338 | |||||
| Petroleum ether (40-60) |
| 15 mL | H225 H304 H315 H336 H411 EUH- | P210 P261 P273 P301 + 310 P331 | |||||
| Water |
| 75 mL | H- EUH- | P- | |||||
| Others | Amount | Risk | Safety | ||||||
| Potassium carbonate |
| ~ 1 g | H302 H315 H319 H335 | P261 P305 + 351 + 338 | |||||
| Magnesium sulfate |
| ~ 1 g | |||||||
| Nitrogen |
| H280 | P410 + 403 | ||||||
| Solvents for analysis | Amount | Risk | Safety | ||||||
| Acetic acid ethyl ester |
| 15 mL | H225 H319 H336 EUH066 | P210 P261 P305 + 351 + 338 | |||||
| Acetonitrile |
| H225 H302 + 312 + 332 H319 | P210 P280 P305 + 351 + 338 | ||||||
| Water |
| H- EUH- | P- |
Substances produced |
| Products | Amount | Risk | Safety | ||
|---|---|---|---|---|---|
| cis-2,2-Dimethyl-4,5-diphenyl-1,3-dioxolane | 0.918 g |
| Waste | Disposal |
|---|---|
| aqueous phase after shaking out | solvent water mixtures, containing halogens |
| evaporated ethyl acetate | organic solvents, halogen free |
| evaporated petroleum ether | solvents for rectification |
Equipment |
Simple evaluation indices |
| Atom economy | 93.4 | % | |
| Yield | 72 | % | |
| Target product mass | 0.918 | g | |
| Sum of input masses | 160 | g | |
| Mass efficiency | 5.6 | mg/g | |
| Mass index | 180 | g input / g product | |
| E factor | 180 | g waste / g product |
Chromatogram |

| HPLC: crude product | |
| column | Phenomenex Luna C18; particle diameter 3 µm, L=150 mm, ID= 4.6 mm |
| column temperature | 25 °C |
| injection | 5 µL |
| mobile phase | 5% MeCN / H2O, gradient to 95% MeCN / H2O (40 min), 10 min isocratic |
| flow | 1.0 mL/min |
| detector (UV 220 nm) | percent concentration calculated from relative peak area |

| HPLC: pure product | |
| column | Phenomenex Luna C18; particle diameter 3 µm, L=150 mm, ID=4.6 mm |
| column temperature | 25 °C |
| injection | 5 µL |
| mobile phase | 5% MeCN/H2O, gradient to 95% MeCN/H2O (40 min), 10 min isocratic |
| flow | 1.0 mL/min |
| detector (UV 220 nm) | percent concentration calculated from relative peak area |
Batam, indonesia
Batam - Wikipedia, the free encyclopedia
https://en.wikipedia.org/?title=Batam
Batam (Jawi: باتم) is an island, municipality (an Indonesian kotamadya), the largest city in the Riau Islands Province of Indonesia, the third largest city in ...


Batam Holiday Inn Resort Getaway
Batam is the nearest Indonesian island to Singapore that is accessible by a 30-minute ferry ride from Singapore. My family stayed at the Batam Holiday Inn Resort for a quick getaway to recharge and unwind.
We got there from a ferry at Harbourfront Singapore to the Waterfront Ferry Terminal in Batam and walked about 5-10 minutes to the hotel. Another way is to take the ferry to Batam city centre and take a 15-20 minute cab ride to the hotel.
Batam Holiday Inn
We got a 2-bedroom suite online for a price that was many times cheaper than the resorts at nearby Bintan island. It came with a small kitchen, a living area with a good range of TV channels. Above is a picture taken from our room.
The main attraction for the kids was the swimming pool. There were three outdoor pools and one indoor lap pool. As this was a “do-nothing” vacation break, we did not do much sightseeing in Batam. Most of the time, my kiddos played at the pool and chilled out at the hotel.
One thing that I liked was the body massage at the Holiday Inn’s Tea Tree Spa. It was so popular even with day-trippers that I only managed to get a slot in the early evening. It was a good thing too because I got the big couple massage room all to myself. The Indonesian massage was good and the aromatherapy was very relaxing. Guests who need to watch over their kids could even do the massage in the hotel room, but I guess it wouldn’t have the spa atmosphere. I would go there just for a body massage again because even with the ferry ride, it would still be cheaper than similar body massages in Singapore.
Click HERE for more details of Batam Holiday Inn Resort.
Eating out in Batam
While the hotel served a good breakfast spread and other meals, we explored other foods in Batam outside the hotel. We found this amazingly cheap and decent local food just a short walk from our hotel.
There were also Padang food at Restoran Sederhana in Batam city centre.
Padang food is a cuisine of the Minangkabau people of Sumatra. Dishes were laid out in front of customers and they charge by what you consume.
Golden Prawn 933 Seafood Restaurant is a huge seafood restaurant that is very popular with tour groups. It had fresh seafood at very reasonable prices.
It was a good thing that we came early before the crowd from tour buses arrived.
Barelang Bridge
The only thing worth sightseeing in Batam is the Barelang Bridge, which is a chain of six bridges that connects Batam with nearby islands. Here’s the view from the first bridge.
Thinking of a short getaway to Batam?
For those in Singapore, who just want to unwind somewhere out of Singapore without spending a lot for flights, or tour coaches, or worrying about driving in Malaysia, Batam is a good choice. Plus it is much cheaper than Bintan. I booked my hotel room from Agoda (Click Holiday Inn Batam).
Other hotels in Batam we considered were:
- Harris Resort Waterfront – it is also located in Waterfront city near Holiday Inn. It has bigger pools and more kids facilities, but we needed to get two rooms for our family, which worked out to be more costly than Holiday Inn.
- Montigo Resorts Nongsa and Turi Beach Resort – Nice resorts but more expensive.

Batam map - indonesia - mapcarta, Batam travel map, with photos and hotels. batam from mapcarta, the interactive map.. Welcome batam center, Online features batam island map batam, rempang and galang islands map.
2295 x 2113 · 411 kB · gif, Batam Map
1600 x 1148 · 431 kB · jpeg, Batam Island Map

568 x 321 · 30 kB · jpeg, Batam Indonesia Map

1600 x 1133 · 332 kB · jpeg, Batam Map Nagoya

500 x 406 · 20 kB · gif, Batam Indonesia Golf Course Map

475 x 226 · 59 kB · gif, Indonesia Map


2295 x 2113 · 411 kB · gif, Batam Map

1600 x 1148 · 431 kB · jpeg, Batam Island Map

568 x 321 · 30 kB · jpeg, Batam Indonesia Map

1600 x 1133 · 332 kB · jpeg, Batam Map Nagoya

500 x 406 · 20 kB · gif, Batam Indonesia Golf Course Map

475 x 226 · 59 kB · gif, Indonesia Map

Batam tourism: batam - tripadvisor, Batam tourism: tripadvisor 11,681 reviews batam hotels, attractions, restaurants making batam resource.. http://www.tripadvisor.com.sg/Tourism-g297717-Batam_Riau_Archipelago_Riau_Islands_Province-Vacations.html Batam map, Find complete information batam island site. http://www.batam.com/about-batam/batam-map.html Peta batam : peta jalan & satellite batam - indonesia, Streetdirectory..id batam maps maps states indonesia featuring details towns, lakes, rivers, places interest, roads, borders , . http://www.streetdirectory.com/indonesia/en/batam/
Batam, Indonesia, is an island and also a city in Riau Islands Province of Indonesia, known for its free trade zone area. It is located 20 km off Singapore's south coast. Where virgin jungle once stood are now whole new towns, mosques, churches, temples and supermarkets, soon to be followed by reservoirs with enough water to supply a population of 800,000 and for industrial use, an airport-to become an international gateway.
1. Maha Vihara Duta Maitreya Temple

Batam, Indonesia, has several Buddhist & Hindu Temples in various locations across the island. The temples have worshippers visiting daily and most temples allow tourists & visitors to come and look around. Maha Vihara Duta Maitreya Buddhist Temple is one of the biggest Buddhist temples in South East Asia and is a major attraction in Batam, Indonesia. The temple is located near to the Batam Center ferry terminal. The main chamber consists of statues of Buddha, and the side chambers are Goddess of Mercy (Guan Yin) and Guan Gong deity. Inside there is a shop sellingBuddhist items and a vegetarian restaurant, offering a variety of vegetarian dishes & fruit drinks.
2. Chocolate House

Batam Chocolate House, Indonesia, is a chocolate heaven for chocolate lovers. They offer more than 100 varieties of chocolate to suit your every mood and desire with very reasonable price. Good as gifts or souvenirs for your loved ones and friends back home.
3. Go-Kart Circuit House

Fast Go Karts race around on a purpose built track, just great for the speed junkies! Test out your skills by racing against the clock or with your team mates. Safety equipment is mandatory for all drivers & is provided by the Circuit, Indonesia. Suitable footwear should be worn (no sandals or flip-flops) and long trousers & shirts are preferred.
4. Flying Fox

Challenge your limits over different obstacles and conquering your fears of height whilst ending your way at the Flying Fox to the ground zero at Batam, Indonesia. Suitable footwear should be worn.
5. Indonesia Minature Park

Indonesia Miniature Park, Indonesia, is a synopsis of Indonesian culture, with virtually all aspects of daily life in Indonesia's 32 provinces encapsulated in separate pavilions with the collections of architecture, and traditions are all depicted impeccably.
6. BengKong Dry Market
BengKong Dry Market, Indonesia, is the place you may able to purchase very fresh dry food stuff in wholesales prices. It is also known to sell local tibits such as tapioca chips, prawn crackers and etc.
7. Golden Prawn 555 Seafood Kelong Restaurant

Golden Prawn, Indonesia, is a fresh seafood restaurant in batam, Indonesia. Golden Prawn 555 and 933 is the best, largest and closest from the golden view hotel. It is the place to for Seafood Lovers to be as they serve hundreds of top dishes with variety choices.
8. Golden Tourist Shop

The souvenir shop is located just inside the entrance to Golden Prawn 933 restaurant, Indonesia, on the left side and inside is a souvenir hunters dream where lots and lots of local assorted stuff for sale. You can Buy famous Indonesian Sea Food Crackers, Indonesian Coffee, other Indonesian Food Stuffs, assorted local handicrafts and woven items, refrigerator magnets, mini wallets and bags, pordelain, pewter and more and the best thing is they accept Singapore Dollars.
9. Bird Nest Shop

The Bird Nest Shop, Indonesia, sells assorted Dried Bird's Nest from cave swiftlet birds are harvested by adventurous pickers from nearby caves along the Riau Islands (since the bird's nest are located high in the caves) and the birds nest are primarily from Bird Saliva and is an aphrodisiac for the chinese.
10. Pondok Wisata Cultural Show

At the Pondok Wisata Cultural Show, Indonesia, you will be entertain by local villagers with their traditional trance dance such as The Roaster Dance. Also, you will get to witness other acts by the locals such as peeling coconut skin using mouth, eating fireballs, eating glass and more.
11. Polo Outlets
Batam Polo Outlet, Indonesia, where you may wish to purchase clothes by American brand Polo Ralph Lauren, which were made in Jakarta, Indonesia, and cheaper than in Singapore. Polo clothing is also available at Batam Center Ferry Terminal, Mega Mall, Nagoya Hill shopping center, the lobby in the Harmoni Hotel and at Batam View Beacch Resort, but the
Shop is the best location for genuine Polo clothing. If you’re on a Batam shopping trip, the Batam Polo Shop is worth a visit!

12. Tua Pek Kong Temple

Tua Pek Kong Temple, Indonesia, is also known as Vihara Budhi Bhakti Temple, this chinese temple is located in Nagoya. It is one of the historical temples in Batam. It is well used by the locals for their daily prayers and offerings. The temple is built in traditional chinese style and has many courtyard statues and several wall paintings.
13. Batam Shopping Mall

Batam Shopping, Indonesia, can be a rewarding experience as there are several large Shopping Malls, as well as many smaller specialty shops selling goods ranging from traditional Indonesian furnitures and handicraft, to electronic and household goods, as well as many clothing and fashion shops.
//////////