Vinyl ethers are important and useful synthetic building blocks. Using a test tube with a screw cap, a convenient preparation of vinyl ethers from alcohols with calcium carbide under superbasic catalytic conditions (KOH/DMSO) was developed. The vinylation of primary and secondary alcohols was successfully achieved, affording the desired products in good yields. The gram-scale preparation of a vinyl ether was also demonstrated. In this reaction, calcium carbide acts as an acetylene source, constituting a safer alternative to acetylene gas.
F. de Nanteuil, E. Serrano, D. Perrotta and J. Waser, J. Am. Chem. Soc., 2014, 136, 6239.
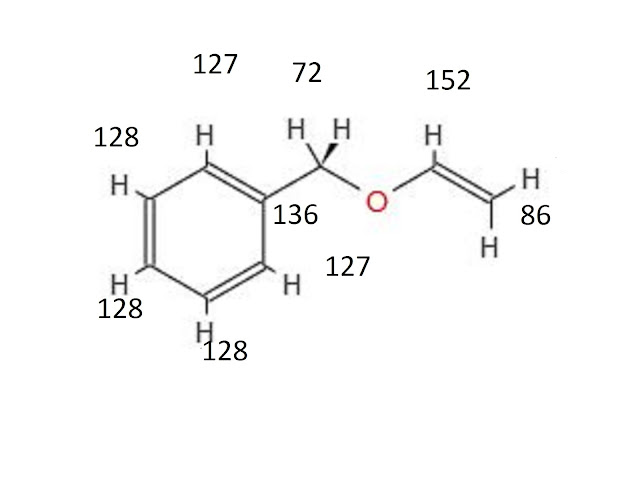
1H NMR
1H NMR PREDICT using nmrdb , signals may vary , use your discretion to understand sequence
13C NMR
13 C NMR PREDICT
Synthesis of vinyl ethers of alcohols using calcium carbide under superbasic catalytic conditions (KOH/DMSO)
*
Corresponding authors
a
Department of Chemistry, Graduate School of Science, Osaka Prefecture University, Sakai, Japan
E-mail: matsu@c.s.osakafu-u.ac.jp
E-mail: matsu@c.s.osakafu-u.ac.jp
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02977E //////////////////// WORLDS BEST BEACHES
1. Le Grottes Plage, Costa Azul, France
Among popular beaches in the globe, Le Grottes Plage is definitely worth a visit. Dotted with restaurants, this beach is a beach lover’s paradise.


2. Little Beach, Maui
Dotted with volcanic rock formations and dense shrubs, this beach is aparadise and is well known as the ‘unofficial clothing optional beach’.


3. Playa Las Suecas, Contadora Island, Panama
Among nudist beaches in the globe, this is an officialcolony where you are allowed to take off your clothing as and when you desire!


4. Lokrum Island, Dubrovnik, Croatia
A haven for those that love to bathe this beach can be reached via boats and jetties. Rocky formations and sandy shore make this beach a paradise among nudists.


5. Haulover Beach, Florida, USA
This beach is counted as number five in this list of most popular beaches in the world because of its exotic beauty that enthralls its bathers that come here to sun bathe in their most natural form.



6. Hedonism II, Negril, Jamaica
With a white sandy shoreline stretching 7 miles, this beach is a holiday maker’s Mecca and is visited, both by couples and families. It’s a passionate place where you can indulge in fulfilling many of your desires! Wink…Wink!


7. Black’s Beach, San Diego, United States
If visiting USA, make a visit to Black’s Beach that is one of most famous and beautiful beaches in the whole of United States. It is also a surfer’s paradise.


8. Montalivet Beach, France
The beach is famous for its beach resort, the very place where international naturist movement started and boasts of 1800 campsites where you can take your clothes off and chill out.

9. Abrico Beach, Rio de Janeiro, Brazil
A bare all beach, it attracts nude bathers from all quarters of the globe. One can take part in a number of fitness programs here.

10. Club Orient, St. Martin, Caribbean
It is a famous resort and is counted among the world’s best beaches simply because of its picturesque location and the presence of casinos and shopping arcades that make it a fun spot for tourists.


11. Arambol Beach, Goa, India
Armabol is a nudist’s favorite beach in India and is dotted with a rocky terrain and smooth waters.


12. Red Beach, Matala, Greece
When writing about world’s best beaches, mention must be made of this exotic beach that used to be a haven for hippies in the late 60’s. Dotted with caves and ruins, this beach surely will enthrall all of you with its pristine beauty.


Image Credit: googleusercontent
13. El Torn Beach, Tarragona, Spain
Most popular among nude activists and lovers, this beach is one of the best beaches in Spain as bathers here can roam around wearing just about nothing, without having to give anyone an explanation!

14. Samurai Beach, Tomaree National Park, Australia
Drive down the beautiful Pacific Motorway, north of the capital city of Sydney to reach this awesome beach that is a hub for nude bathers that come here to play sports like volleyball and tug of war.

15. Plakias Beach, Crete, Greece
Plakias is Crete Island’s biggest beach that attracts bathers and scuba divers from all across the planet.

16. Praia do Pinho Beach, Brazil
This beach is located in Santa Catarina and opened its doors to the nudists way back in 1987. From this beach, you can visit the world famous stature of Christ known as Cristo Luz.

Image Credit: travelchannel

17. Wreck Beach, Vancouver, Canada
The beautiful beach stretches to more than 7.8 km in length and is one of the world’s best beaches that is now counted as one of the seventh wonders of Canadaaaronvonhagen.


18. Spiaggia di Guvano, Corniglia, Italy
this beach can be reached once you cross the many caves that flank the shores.


19. Baker Beach, San Francisco, USA
San Francisco isn’t merely famous for its Golden Bridge, but attracts visitor to its rocky beach that offers majestic views of the bridge and is also a heaven for nude bathers. But it is only towards the end of the northern portion of this beach that you can sun bathe in the nude.



20. Moshup Beach, Massachusetts, USA
Known for its Bohemian styled sun bathers that come here to bathe and tan, this beach is accessible to nudists as well as others.


//////