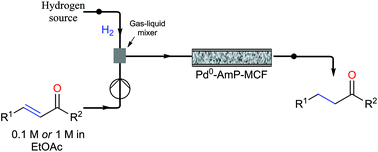
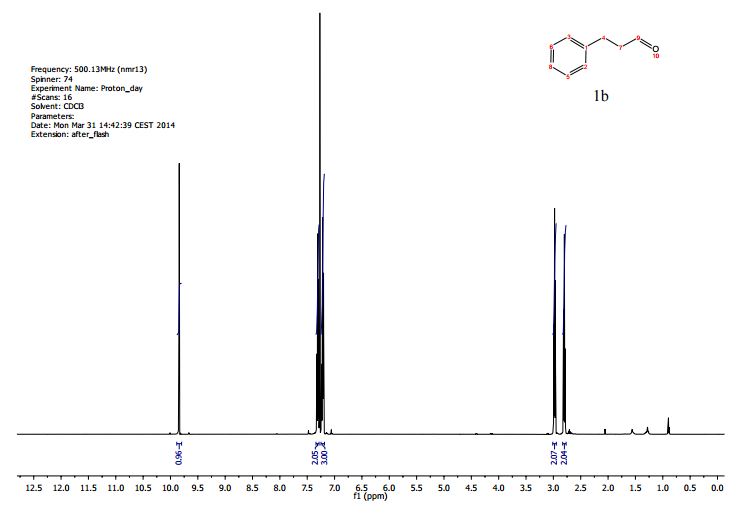
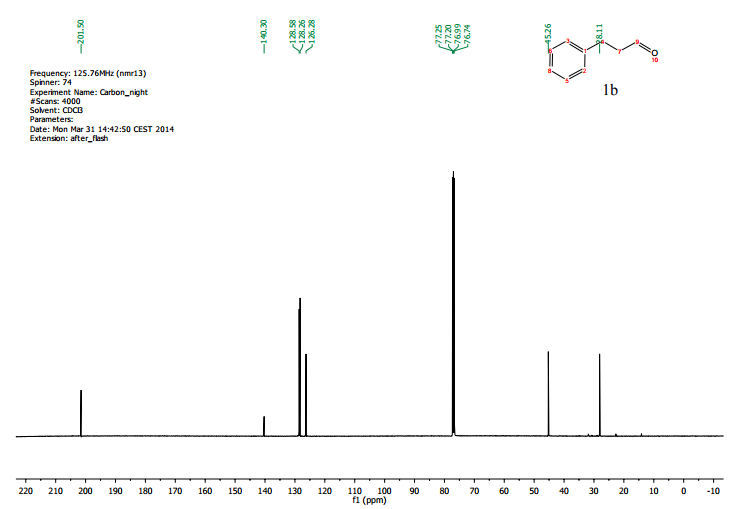
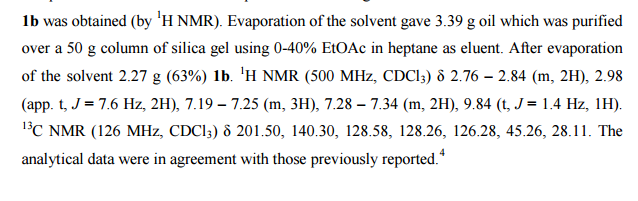
.
.Methyl (2S,4R)-1-benzyl-4-hydroxypyrrolidine-2-carboxylate
Toluene (30 mL) was added to a round bottom flask and (2S,4R)-4-hydroxy-2-(methoxycarbonyl)pyrrolidin-1-ium chloride (5.30 g, 29.2 mmol) was suspended. The mixture was cooled down to 0°C. Firstly N,N-Diisopropylethylamine (DIPEA, 12.60 mL, 72.34 mmol, 2.48 equiv.) was added drop wise and then followed by benzyl bromide (3.80 mL, 31.92 mmol, 1.09 equiv.). The reaction mixture was refluxed for 6 h and quenched with saturated aqueous ammonium chloride solution (30 mL). The aqueous solution was extracted with ethyl acetate (3 × 150 mL). The combined organic layers were washed with brine (150 mL) and dried over sodium sulfate. Filtering of the drying agent and solvent removal under reduced pressure gave the product (6.74 g, 28.7 mmol, 98% yield) as yellowish oil. 1H-NMR (CDCl3, 300 MHz): δ = 1.75 (1H, br-s), 2.02–2,13 (1H, m), 2.18–2.33 (1H, m), 2.48 (1H, dd, J = 10.2, 3.7 Hz), 3.33 (1H, dd, J = 10.2, 5.6 Hz), 3.58–3.70 (2H, m), 3.66 (3H, s), 3.90 (1H, d, 12.9 Hz), 4.45 (1H, br-s), 7.20–7.38 (5H, m) ppm. 13C-NMR (CDCl3, 75 MHz): δ = 39.6,51.9, 58.2, 61.3, 63.7, 70.4, 127.36, 128.4, 129.2, 138.2, 174.1 ppm. Analytic data agree with literature [v].
Alza E, Sayalero S, Kasaplar P, Almasi D, Pericas MA (2011) Polystyrene supported diarylprolinol ethers as highly efficient organocatalysts for Michael-type reactions. Chem Eur J 17:11585–11595View Article
///////
Oludeniz beach, Turkey

 Oludeniz is Turkey's top beach destination and understandably is the
most crowded. It is located on the south -west coast of Turkey in
Fethiya town.
Oludeniz is Turkey's top beach destination and understandably is the
most crowded. It is located on the south -west coast of Turkey in
Fethiya town. Famously known as the Turquoise Coast, the water here is an
enchanting blue, and because the curved beach is so large, it can
accommodate even the biggest crowds. There is beautiful mountain scenery
and a blue lagoon with a large underwater park along the beach's
western stretch.
Famously known as the Turquoise Coast, the water here is an
enchanting blue, and because the curved beach is so large, it can
accommodate even the biggest crowds. There is beautiful mountain scenery
and a blue lagoon with a large underwater park along the beach's
western stretch.
The beach is surrounded by many resorts, especially the main hillside town of Belcekiz, which has crystal clear water, and a long shingle beach curving away from the town promenade. There are plenty of restaurants and caf�s where umbrellas and loungers can be hired. There are good hotels to stay at with added attractions like scuba diving; paragliding and hang gliding.
There are many other attractions at Olu Deniz like the picturesque Butterfly Valley, Saklikent Gorge, St Nicolas Island, or the Lycian Way.
Best time to visit Olu Deniz is from May to Oct. Summer temperature averages 32C while winters are pleasant with temperatures around 20C.
Dalaman about 30 miles away, is the closest airport. Local buses and taxis are available from the town of Fethiye which is seven miles away.







////////
Oludeniz beach, Turkey
Oludeniz Beach
The beach is surrounded by many resorts, especially the main hillside town of Belcekiz, which has crystal clear water, and a long shingle beach curving away from the town promenade. There are plenty of restaurants and caf�s where umbrellas and loungers can be hired. There are good hotels to stay at with added attractions like scuba diving; paragliding and hang gliding.
There are many other attractions at Olu Deniz like the picturesque Butterfly Valley, Saklikent Gorge, St Nicolas Island, or the Lycian Way.
Best time to visit Olu Deniz is from May to Oct. Summer temperature averages 32C while winters are pleasant with temperatures around 20C.
Dalaman about 30 miles away, is the closest airport. Local buses and taxis are available from the town of Fethiye which is seven miles away.

////////