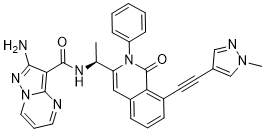
IPI-549
CAS 1693758-51-8
MF : C30H24N8O2
Molecular Weight: 528.576
MF : C30H24N8O2
Molecular Weight: 528.576
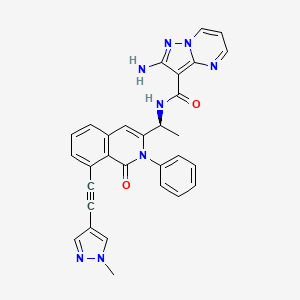
(S)-2-amino-N-(1-(8-((1-methyl-1H-pyrazol-4-yl)ethynyl)-1-oxo-2-phenyl-1,2-dihydroisoquinolin-3-yl)ethyl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
| Company | Infinity Pharmaceuticals Inc. |
| Description | Small molecule inhibitor of phosphoinositide 3-kinase (PI3K) gamma |
| Molecular Target | Phosphoinositide 3-kinase (PI3K) gamma |
| Mechanism of Action | Phosphoinositide 3-kinase (PI3K) gamma inhibitor |
| Therapeutic Modality | Small molecule |
| Latest Stage of Development | Phase I |
| Standard Indication | Solid tumors |
| Indication Details | Treat solid tumors |
- Originator Intellikine
- Developer Infinity Pharmaceuticals
- ClassAntineoplastics; Small molecules
- Mechanism of ActionPhosphatidylinositol 3 kinase delta inhibitors; Phosphatidylinositol 3 kinase gamma inhibitors
- Phase I Solid tumours

Most Recent Events
- 18 Apr 2016 Pharmacodynamics data from a preclinical study in Solid tumours presented at the 107th Annual Meeting of the American Association for Cancer Research (AACR-2016)
- 01 Dec 2015 Phase-I clinical trials in Solid tumours (Monotherapy, Combination therapy, Late-stage disease, Second-line therapy or greater) in USA (PO)
IPI-549 is a potent and selective phosphoinositide-3-kinase (PI3Kγ) Inhibitor as an Immuno-Oncology Clinical Candidate (Kd = 0.29 nM). Bioactivity data of IPI-549: biochemcial IC50 (nM) for PI3K isoform: 3200 (α); 3500 (β); 16 (γ); and >8400 (δ) respectively. Cellar IC50 (nM) of IPI549 for PI3K isoform: 250 (α); 240 (β); 1.6 (γ); and 180 (δ) respectively. IPI-549 shows >100-fold selectivity over other lipid and protein kinases. IPI-549 demonstrates favorable pharmacokinetic properties and robust inhibition of PI3K-γ mediated neutrophil migration in vivo and is currently in Phase 1 clinical evaluation in subjects with advanced solid tumors.

Patent
WO 2015051244
Scheme 1

Scheme 2

Example 1

[00657] Compound 4 was prepared in 3 steps from compound A according to the following procedures:
Compound A was prepared according to Method A. It was coupled to 2-((tert-butoxycarbonyl)amino)pyrazolo[l,5-a]pyrimidine-3-carboxylic acid according to the following procedure: Compound A (27.4 mmol, 1.0 equiv), HOBt hydrate (1.2 equiv), 2-((tert-butoxycarbonyl)amino)pyrazolo[l,5-a]pyrimidine-3-carboxylic acid (1.05 equiv) and
EDC (1.25 equiv) were added to a 200 mL round bottomed flask with a stir bar. N,N-Dimethylformamide (50 mL) was added and the suspension was stirred at RT for 2 min. Hunig's base (4.0 equiv) was added and after which the suspension became homogeneous and was stirred for 22h resulting in the formation of a solid cake in the reaction flask. The solid mixture was added to water (600 mL) and stirred for 3h. The resulting cream colored solid was filtered and washed with water (2 x 100 mL) and dried. The solid was then dissolved in methylene chloride (40 mL) after which trifluoroacetic acid (10 equiv, 20 mL) was added and the reaction was stirred for 30 min at RT after which there is no more starting material by LC/MS analysis. The solution was then concentrated and coevaporated with a mixture of methylene choride/ethanol (1 : 1 v/v) and then dried under high vacuum overnight. The resulting solid was triturated with 60 mL of ethanol for lh and then collected via vacuum filtration. The beige solid was then neutralized with sodium carbonate solution (100 mL) and then transferred to a separatory funnel with methylene chloride (350 mL). The water layer was extracted with an additional 100 mL of methylene chloride. The combined organic layers were dried over sodium sulfate, filtered and concentrated under vacuum to provide a pale yellow solid that was purified using flash silica gel chromatography (Combiflash, 24g column, gradient of 0-5% methanol/methylene chloride) to provide amide B. ESI-MS m/z: 459.4 [M+H]+.
[00658] Amide B was placed in a sealed tube (0.67 mmol, 1.0 equiv) followed by dichlorobis(acetonitrile)palladium (15 mol%), X-Phos (45 mol%), and cesium carbonate (3.0 equiv) Propionitrile (5 mL) was added and the mixture was bubbled with Ar for 1 min. 4-Ethynyl-l -methyl- lH-pyrazole (1.24 equiv) was added and the resulting orange mixture was sealed and stirred in an oil bath at 85 oC for 1.5h. The resulting brownish-black mixture was allowed to cool at which point there was no more SM by LC/MS analysis. The mixture was then filtered through a short plug of cotton using acetonitrile and methylene chloride. The combined filtrates were concentrated onto silica gel and purified using flash silica gel chromatography (Combiflash, 4g column, gradient of 0-5% methylene chloride/methanol). The resulting material was further purified by reverse phase HPLC (15-90%o acetonitrile with 0.1%o formic acid/water with 0.1%o formic water) to provide desired compound 4. ESI-MS m/z: 529.5 [M+H]+.
PAPER
WO 2015143012
PAPER
IPI-549 NMR 1H
IPI-549 13C NMR
IPI-549 ASSAY
Compound 1 is coupled to 4-ethynyl-1-methyl-1H-pyrazole using the general procedure outlined above to provide compound 26 IPI-549, in 70% yield with >98% enantiomeric purity.
IPI-549
1H NMR (400 MHz, DMSO-d6) δ 8.92 (dd, J = 6.8, 1.7 Hz, 1H), 8.55 (dd, J = 4.5, 1.7 Hz, 1H), 8.00 (d, J=6.8 Hz, 1H), 8.00 (s, 1H), 7.69 – 7.54 (m, 5H), 7.53 – 7.43 (m, 3H), 7.41 – 7.35 (m, 1H), 7.01 (dd, J = 6.7, 4.5 Hz, 1H), 6.74 (s, 1H), 6.42 (s, 2H), 4.56 (quin, J = 6.8 Hz, 1H).), 3.82 (s, 3H), 1.35 (d, J = 6.8 Hz, 3H).
13C NMR (101 MHz, DMSO-d6) δ 162.73, 161.19, 160.93, 150.06, 147.51, 146.74, 141.05, 138.09, 137.81, 135.42, 133.66, 132.56, 131.90, 129.51, 129.24, 129.20, 129.17, 128.50, 126.16, 123.41, 123.31, 107.88, 102.44, 101.15, 90.40, 87.06, 85.94, 44.88, 38.62, 20.69.
ESI-HRMS: calcd for 529.2095 C30H25N8O2 (M+H)+ , found 529.2148.
[]D 22: +447.8o (c 1.007, DMSO)
COMPD1
compound 1 in 95% yield.
1H NMR (400 MHz, CDCl3) 8.41 (dd, J = 6.8, 1.7 Hz, 1H), 8.37 (dd, J = 4.4, 1.7 Hz, 1H), 7.90 (d, J = 7.0 Hz, 1H), 7.50-7.34 (m, 5H), 7.34-7.27 (m, 2H), 6.76 (dd, J = 7.1, 4.9 Hz, 1H), 6.57 (s, 1H), 5.54 (broad s, 2H), 4.79 (quin, J = 6.9 Hz, 1H), 1.36 (d, J = 6.5 Hz, 3H);
ESI-HRMS: calcd for C24H20ClN6O2 459.1331 (M+H)+ , found 459.1386. HPLC Purity: 96% AUC.

Optimization of isoquinolinone PI3K inhibitors led to the discovery of a potent inhibitor of PI3K-γ (26 or IPI-549) with >100-fold selectivity over other lipid and protein kinases. IPI-549 demonstrates favorable pharmacokinetic properties and robust inhibition of PI3K-γ mediated neutrophil migration in vivo and is currently in Phase 1 clinical evaluation in subjects with advanced solid tumors.
Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate
Infinity Pharmaceuticals, Inc., 784 Memorial Drive, Cambridge, Massachusetts 02139, United States
ACS Med. Chem. Lett., 2016, 7 (9), pp 862–867
DOI: 10.1021/acsmedchemlett.6b00238
*E-mail: catherine.evans@infi.com., *E-mail: alfredo.castro@infi.com.

CLIP
Infinity Expands Pipeline with Addition of IPI-549, an Immuno-Oncology Development Candidate for the Treatment of Solid Tumors
– IPI-549, a Selective PI3K-Gamma Inhibitor, Targets the Immune-Suppressive Tumor Microenvironment –
– Preclinical Data for IPI-549 Presented at CRI-CIMT-EATI-AACR - The Inaugural International Cancer Immunotherapy Conference –
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Infinity Pharmaceuticals, Inc. (NASDAQ: INFI) today announced the expansion of its pipeline with the addition of IPI-549, an orally administered immuno-oncology development candidate that selectively inhibits phosphoinositide-3-kinase gamma (PI3K-gamma), for the treatment of solid tumors. Preclinical data demonstrating the potential of IPI-549 to disrupt the immune-suppressive tumor microenvironment and enable a heightened anti-tumor immune response are being presented today at CRI-CIMT-EATI-AACR - The Inaugural International Cancer Immunotherapy Conference: Translating Science into Survival Meeting in New York City. IPI-549 was discovered at Infinity and is expected to enter Phase 1 clinical development in early 2016.
“Infinity is committed to developing first-in-class and best-in-class medicines, and the expansion of our pipeline with the addition of IPI-549 represents an important step toward fulfilling our vision of building a sustainable biopharmaceutical company that brings meaningful medicines to patients”Tweet this
“Infinity is committed to developing first-in-class and best-in-class medicines, and the expansion of our pipeline with the addition of IPI-549 represents an important step toward fulfilling our vision of building a sustainable biopharmaceutical company that brings meaningful medicines to patients,” stated Vito Palombella, Ph.D., Infinity’s chief scientific officer. “Infinity’s ability to internally develop a selective PI3K-gamma inhibitor provides us with a unique opportunity to explore the impact that PI3K-gamma inhibition has on disrupting the tumor microenvironment. We look forward to initiating the first clinical study of IPI-549 in patients with solid tumors.”
“I have had the pleasure of collaborating with Infinity’s discovery team and am excited to have worked with IPI-549 in my laboratory,” Jedd Wolchok, M.D., Ph.D., chief of Melanoma and Immunotherapeutics Service, Lloyd J. Old/Ludwig Chair in Clinical Investigation Department of Medicine and Ludwig Center, at Memorial Sloan Kettering Cancer Center and the principal investigator for the planned Phase 1 clinical study of IPI-549. “IPI-549 is a novel, small molecule immuno-oncology agent, and I am looking forward to leading the Phase 1 study for this program.”
IPI-549 inhibits immune suppressive macrophages within the tumor microenvironment, whereas other immunotherapies such as checkpoint modulators more directly target immune effector cell function. As such, IPI-549 may have the potential to treat a broad range of solid tumors and represents a potentially complementary approach to restoring anti-tumor immunity in combination with other immunotherapies such as checkpoint inhibitors.
Preclinical Data for IPI-549 Presented at CRI-CIMT-EATI-AACR - The Inaugural International Cancer Immunotherapy Conference
Today at the AACR meeting in New York City Infinity researchers are presenting preclinical data for IPI-549 in a poster entitled, “The potent and selective phosphoinositide-3-kinase-gamma inhibitor, IPI-549, inhibits tumor growth in murine syngeneic solid tumor models through alterations in the immune suppressive microenvironment.”
In vitro data showed that IPI-549 blocks both the migration of murine myeloid cells and the differentiation of myeloid cells to the M2 phenotype, which is a type of myeloid cell known to promote cancer growth and suppress anti-tumor immune responses. In vivo data in murine solid tumor models demonstrated that IPI-549 treatment also decreased tumor-associated myeloid cells found in the immune suppressive microenvironment. Additionally, IPI-549 treatment increased the number of intratumoral CD8+T-cells, which are known to play a role in inhibiting tumor growth.
IPI-549 has demonstrated dose-dependent, single-agent, anti-tumor activity in multiple solid tumor models, including murine models of lung, colon and breast cancer. Additionally, mice treated with IPI-549 in combination with checkpoint inhibitors showed greater tumor growth inhibition than either treatment as a monotherapy. Preclinical in vivo data also demonstrated that T-cells are required for the anti-tumor activity of IPI-549, which is a hallmark of immunotherapy.
Further details about the IPI-549 development program will be provided at Infinity’s R&D Day on Tuesday, October 6, 2015. R&D Day will be held in New York City from 7:30 a.m. to 12:00 p.m. ET. The event will be webcast beginning at 8:00 a.m. ET and can be accessed in the Investors/Media section of Infinity’s website, www.infi.com. A replay of the event will also be available.
Infinity is also developing duvelisib, an investigational, oral, dual inhibitor of PI3K-delta and PI3K-gamma. The PI3K pathway is also known to play a critical role in regulating the growth and survival of certain types of blood cancers. The investigational agent is being evaluated in registration-focused studies, including DYNAMOTM, a Phase 2 study in patients with refractory indolent non-Hodgkin lymphoma, DYNAMO+R, a Phase 3 study in patients with previously treated follicular lymphoma, and DUOTM, a Phase 3 study in patients with relapsed/refractory chronic lymphocytic leukemia. Duvelisib is an investigational compound and its safety and efficacy have not been evaluated by the U.S. Food and Drug Administration or any other health authority.
About Infinity Pharmaceuticals, Inc.
Infinity is an innovative biopharmaceutical company dedicated to discovering, developing and delivering best-in-class medicines to people with difficult-to-treat diseases. Infinity combines proven scientific expertise with a passion for developing novel small molecule drugs that target emerging disease pathways. For more information on Infinity, please refer to the company’s website at www.infi.com.
Clip
IPI-549-01-A phase 1/1b first in human study of IPI-549, a PI3K-γ inhibitor, as monotherapy and in combination with pembrolizumab in subjects with advanced solid tumors.
Subcategory:
Category:
Developmental Therapeutics—Immunotherapy
Meeting:
Session Type and Session Title:
Poster Session, Developmental Therapeutics—Immunotherapy
Abstract Number: TPS3111
Poster Board Number:
Board #425a
Citation:
J Clin Oncol 34, 2016 (suppl; abstr TPS3111)
Abstract:
Background: IPI-549 is a potential first-in-class potent and selective PI3K-γ inhibitor being developed as a novel orally administered immuno-oncology therapeutic in multiple cancer indications. Preclinical studies demonstrate a role for PI3K-γ in polarization of immune suppressive myeloid cells in the tumor microenvironment. Inhibition of PI3K-γ by IPI-549 enhanced antitumor immune responses and inhibited tumor growth in syngeneic solid tumor preclinical models. In addition, IPI-549 in combination with immune checkpoint inhibitors showed increased tumor growth inhibition compared to each single agent in multiple pre-clinical models. These data served as the scientific foundation for initiating a clinical trial testing IPI-549 as a potential immuno-oncology therapy. This first-in-human clinical study will evaluate the safety and tolerability, and determine the recommended Phase 2 dose (RP2D) of IPI-549 when administered as a monotherapy and in combination with pembrolizumab (NCT02637531) in solid tumors. Methods: This multi-part Phase 1/1b open-label trial will initiate with monotherapy dose escalation cohorts consisting of an accelerated dose escalation phase followed by a standard phase with a 3+3 design. Evaluation of the PK, PD, and safety data in these cohorts will lead to the determination of the maximum tolerated dose (MTD) and RP2D of IPI-549 monotherapy. Subsequently, combination dose escalation cohorts will be initiated in which the combination of IPI-549 and pembrolizumab will be evaluated. Expansion cohorts evaluating the safety, PK, PD, and preliminary clinical activity of IPI-549 as a monotherapy and in combination with pembrolizumab will occur following the dose escalation phase. All subjects in the trial will have advanced and/or metastatic carcinoma or melanoma, and will have failed to respond to standard therapies. Combination expansion cohorts will recruit subjects with non-small cell lung cancer or melanoma who must have received an anti-PD-1 or anti-PD-L1 antibody as their most recent treatment. This trial is currently enrolling patients in the US. Clinical trial information: NCT02637531
REFERENCES
Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate
Catherine A. Evans, Tao Liu, André Lescarbeau, Somarajan J. Nair, Louis Grenier, Johan A. Pradeilles, Quentin Glenadel, Thomas Tibbitts, Ann M. Rowley, Jonathan P. DiNitto, Erin E. Brophy, Erin L. O’Hearn, Janid A. Ali, David G. Winkler, Stanley I. Goldstein, Patrick O’Hearn, Christian M. Martin, Jennifer G. Hoyt, John R. Soglia, Culver Cheung, Melissa M. Pink, Jennifer L. Proctor, Vito J. Palombella, Martin R. Tremblay, and Alfredo C. Castro
Publication Date (Web): July 22, 2016 (Letter)
DOI: 10.1021/acsmedchemlett.6b00238
Catherine A. Evans, Tao Liu, André Lescarbeau, Somarajan J. Nair, Louis Grenier, Johan A. Pradeilles, Quentin Glenadel, Thomas Tibbitts, Ann M. Rowley, Jonathan P. DiNitto, Erin E. Brophy, Erin L. O’Hearn, Janid A. Ali, David G. Winkler, Stanley I. Goldstein, Patrick O’Hearn, Christian M. Martin, Jennifer G. Hoyt, John R. Soglia, Culver Cheung, Melissa M. Pink, Jennifer L. Proctor, Vito J. Palombella, Martin R. Tremblay, and Alfredo C. Castro
Publication Date (Web): July 22, 2016 (Letter)
DOI: 10.1021/acsmedchemlett.6b00238
///////immuno-oncology, IPI-549, isoform selectivity, neutrophil migration, phosphoinositide-3-kinase, PI3K-gamma inhibitor, IPI 549, IPI549.
O=C1N(C2=CC=CC=C2)C([C@@H](NC(C3=C(N=CC=C4)N4N=C3N)=O)C)=CC5=CC=CC(C#CC6=CN(C)N=C6)=C51
///////







 .
.







