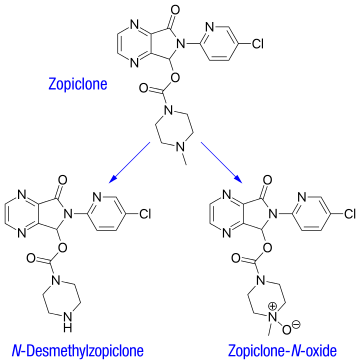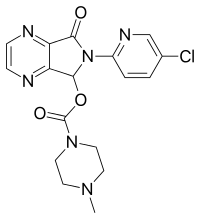
ZOPICLONE
зопиклон
زوبيكلون
佐匹克隆
(±)-Zopiclone
1-Piperazinecarboxy
256-138-9 [EINECS]
43200-80-2 [RN]
Structural formula
UV- Spectrum
IR - spectrum
MASS spectrum
References
- UV and IR Spectra. H.-W. Dibbern, R.M. Muller, E. Wirbitzki, 2002 ECV
- NIST/EPA/NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman. Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
Brief background information
| Salt | ATC | formula | MM | CASE |
|---|---|---|---|---|
| - | N05CF01 | C 17 H 17 ClN 6 O 3 | 388.82 g / mol | 43200-80-2 |
Application
- sedative
- hypnotic
Classes substance
- chlorine compounds
- oxo
- Esters of 1-piperazinecarboxylate
- pyridines
- Pirrolo [3,4-b] piraziny
Synthesis Way
Trade names
| country | Tradename | Manufacturer |
|---|---|---|
| Germany | Optydorm | DOLORGIET |
| Somnosan | Hormos | |
| Ksimovan | Sanofi-Aventis | |
| Zop | HEXAL | |
| Zopi-cigar | Actavis | |
| various generic drugs | ||
| France | imovane | SanofiAventis |
| Noktireks | Sanofi-Synthélabo | |
| United Kingdom | Snowman | SanofiAventis |
| Italy | imovane | SanofiAventis |
| tion | THERE | |
| Japan | Amoʙan | Sanofi-Aventis; Chugai; Mitsubishi |
| Ukraine | imovane | Sanofi Winthrop Indastria, France |
| various generic drugs | ||
Formulations
- coated tablets 7.5 mg;
- Tablets 7.5 mg, 10 mg
References
- DOS 2 300 491 (Rhône-Poulenc; appl. 5.1.1973; F-prior. 7.1.1972, 9.9.1972).
- US 3 862 149 (Rhône-Poulenc; 21.1.1975; F-prior. 7.1.1972, 9.9.1972).
| CAS Registry No.: | 43200-80-2 | ||
| Molecular Formula: | C17H17ClN6O3 | Molecular Weight: | 388.8 |
| Compound Name: | |||
| zopiclone 1-piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl ester 4-methyl-1-piperazinecarboxylic acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)-pyrazin-5-yl ester 4-methyl-1-piperazinecarboxylic acid-6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)-pyrazin-5-yl ester 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl 4-methyl-1-piperazinecarboxylate 6-(5-chloro-pyridin-2-yl)-5((4-methyl-1-piperazinyl)carbonyloxy)-7-oxo-6,7-dihydro-5H-pyrrolo(3,4-b)pyrazine 6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo(3,4-b)pyrazin-5-yl 4-methylpiperazine-1-carboxylate amoban (R) imovane | |||
Zopiclone (Imovance), 4-methyl-1-piperzinecarboxylic acid 6-(5-chloro-2- pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester (Figure 1) is one of the non benzodiazepine sedative-hypnotics of the cyclopyrrolone class, sold by Rhone-Poulene Company in France since 1987. Although structurally unrelated to benzodiazepines, its pharmacological profile is similar, exhibiting sedative-hypnotic, anxiolytic, myorelaxant, and anticonvulsant activity.[1] Other than the first generation barbiturates and the second-generation benzodiazepines, zopiclone, which is widely used in Europe as well as other regions worldwide,[2,3] as a representative of the third generation sedative-hypnotic drugs, has been shown to be free from residual effects on performance and psychological function the day after intake and from the risks of accumulation because of its short elimination half-life (3.5 to 6.5 hours).[3,4] It is indicated for the short term treatment of insomnia, transient, situational or chronic insomnia, and insomnia secondary to psychiatric disturbances.[3]
REFERENCES 1. Mann, K.; Bauer, H.; Hiemke, C.; Ro¨schke, J.; Wetzel, H.; Benkert, O. Acute, subchronic and discontinuation effects of zopiclone on sleep EEG and nocturnal melatonin secretion. Eur. Neuropsychopharm. 1996, 6 (3), 163– 168. Structure Elucidation of Sedative-Hypnotic Zopiclone 359 Downloaded by [Dalhousie University] at 22:10 19 December 2012
2. Le´ger, D.; Janus, C.; Pellois, A.; Quera-Salva, M.A.; Dreyfus, J.P. Sleep, morning alertness and quality of life in subjects treated with zopiclone and in good sleepers. study comparing 167 patients and 381 good sleepers. Eur. Psychiat. 1995, 10 (973) Suppl. 3, 99s – 102s.
3. Piperaki, S.; Parissi-Poulou, M. Enantiomeric separation of zopiclone, its metabolites and products of degradation on a b-cyclodextrin bonded phase. J. Chromatogr. A 1996, 729 (1 – 2), 19 – 28
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
(RS)-6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate
| |
| Clinical data | |
| Trade names | Imovane, Zimovane |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category | |
| Routes of administration | Oral tablets, 3.75 mg (UK), 5 or 7.5 mg |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 75-80%[1] |
| Protein binding | 52–59% |
| Metabolism | Hepatic through CYP3A4and CYP2E1 |
| Biological half-life | ~5 hours (3.5–6.5 hours) ~7–9 hours for over 65 |
| Excretion | Urine (80%) |
| Identifiers | |
| CAS Number | 43200-80-2 |
| ATC code | N05CF01 (WHO) |
| PubChem | CID 5735 |
| IUPHAR/BPS | 7430 |
| DrugBank | DB01198 |
| ChemSpider | 5533 |
| UNII | 03A5ORL08Q |
| KEGG | D01372 |
| ChEBI | CHEBI:32315 |
| ChEMBL | CHEMBL135400 |
| PDB ligand ID | ZPC (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C17H17ClN6O3 |
| Molar mass | 388.808 g/mol |
| 3D model (Jmol) | Interactive image |
////////////