 Dedicated to all moms in the world
Dedicated to all moms in the world
C=O group is dad
O atom is mom
Carbonyl is dad and oxygen mom hence c labelled methyl has higher chemical shift and gets a little more attention
SEE BELOW
NMR IS EASY
A chemical has Formula: C5H10O2
C5H10O2
Rule 2, omit O, gives C5H10
5 - 10/2 + 1 = 1 degree of unsaturation.
Look for 1 pi bond or aliphatic ring.
Rule 2, omit O, gives C5H10
5 - 10/2 + 1 = 1 degree of unsaturation.
Look for 1 pi bond or aliphatic ring.
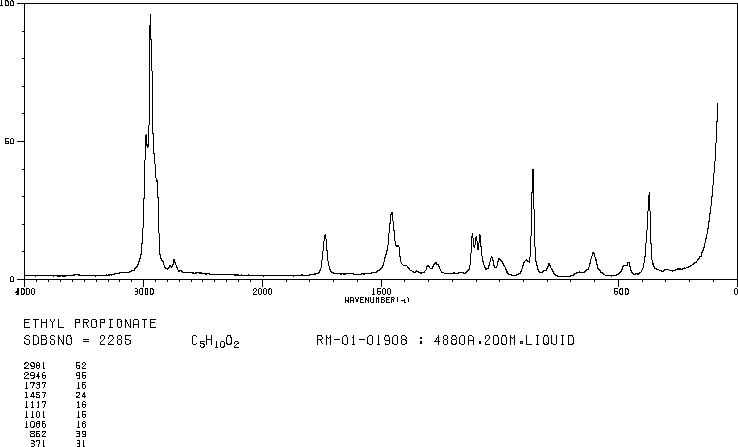
IR
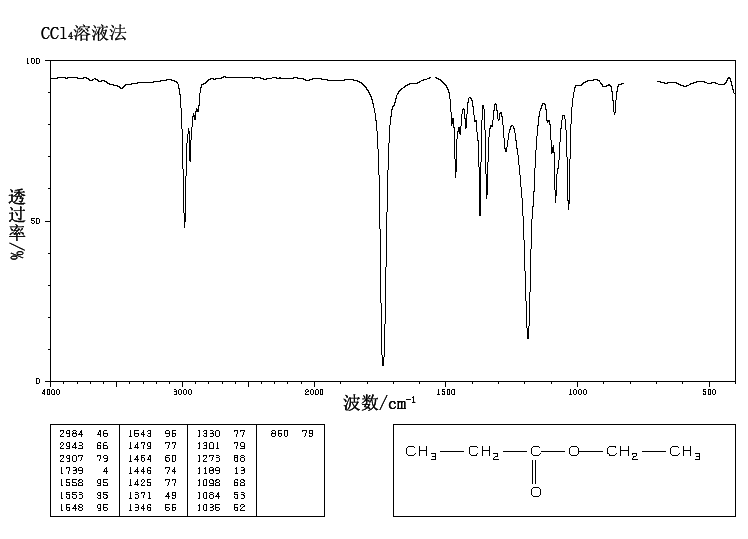
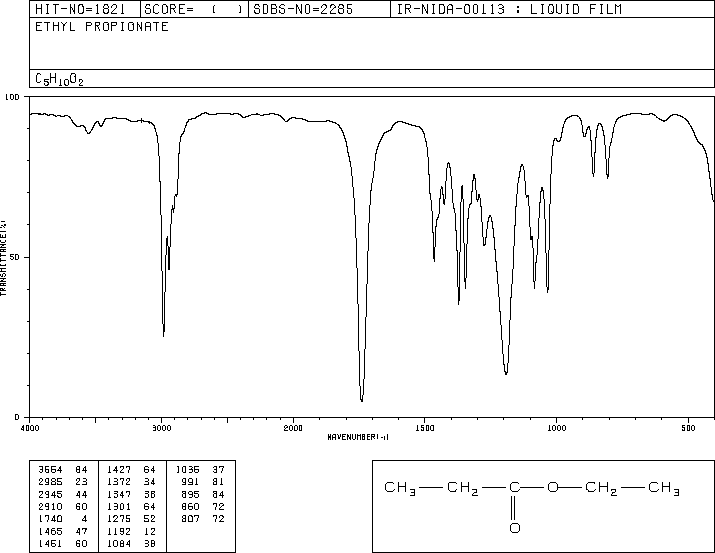

The band at 1740 indicates a carbonyl, probably a saturated aliphatic ester. The bands at 3000-2850 indicate C-H alkane stretches. The bands in the region 1320-1000 could be due to C-O stretch, consistent with an ester.

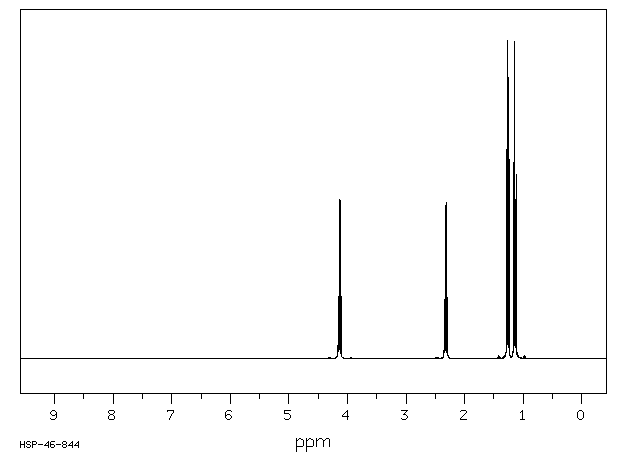
 This is the structure. See if you can assign the peaks on your own.
This is the structure. See if you can assign the peaks on your own. C has a higher chemical shift than D because it's closer to a more electron-withdrawing functional group.
C has a higher chemical shift than D because it's closer to a more electron-withdrawing functional group.
Carbonyl is dad and oxygen mom, hence c has higher chemical shift and gets a little more attention in proton nmr
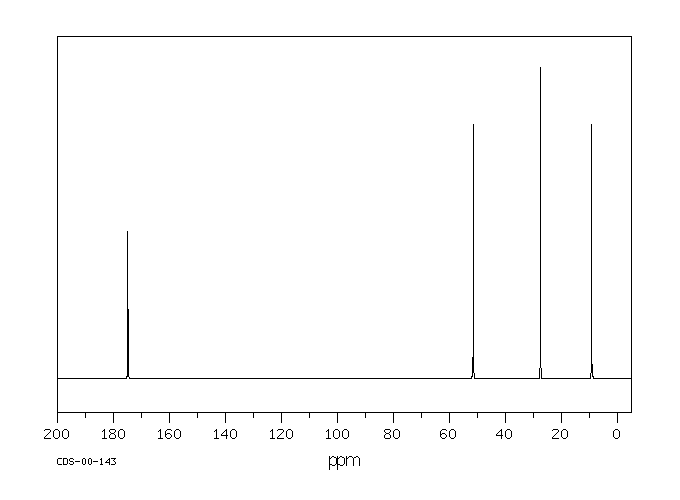
13 C NMR

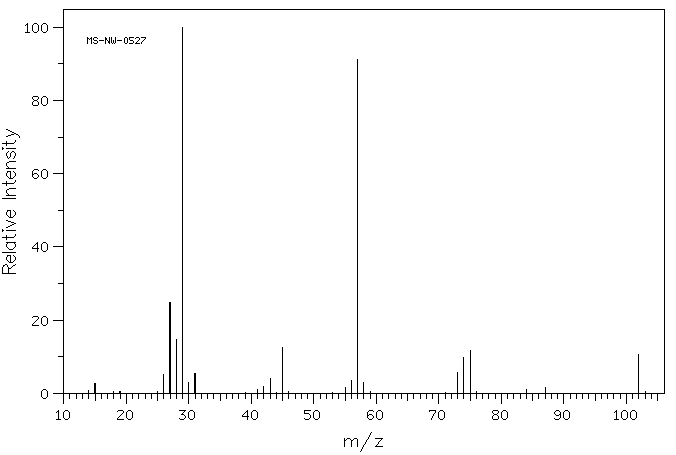
Mass spectrum
RAMAN
WHAT HAPPENS WHEN A CHLORO IS INTRODUCED

THE INTERPRETATION IS BELOW
WHEN THERE IS ONE METHYL

WHEN THERE ONE CH2 SHORT


WHEN MOM HAS ONE MORE CH2
PROPYL PROPIONATE, try this on your own

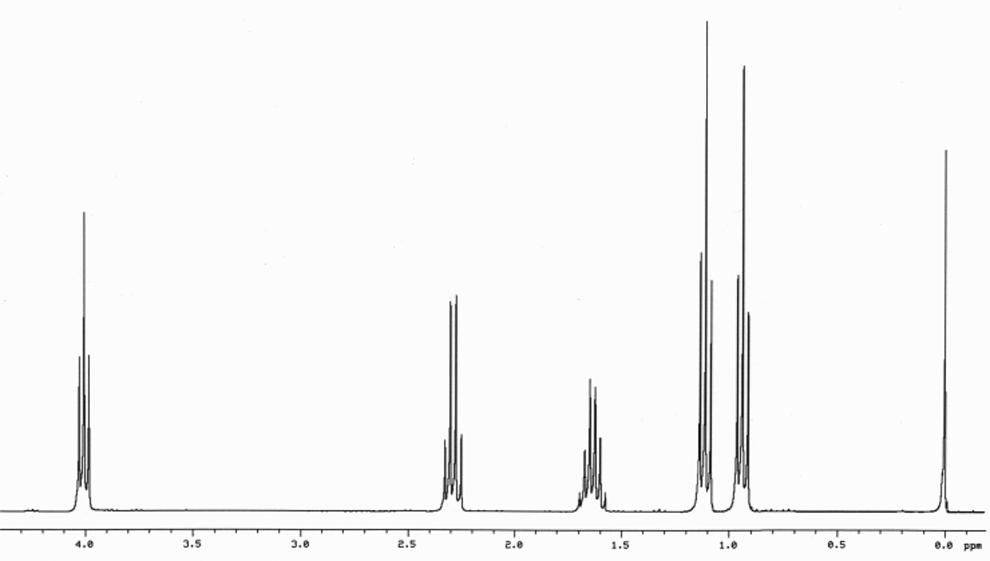
1H NMR
see interpretation
BIGGER ONE THAN OBOVE

13C NMR

APT

COSY

WILL PASTE INTERPRETATION AFTER ONE WEEK...................


No comments:
Post a Comment