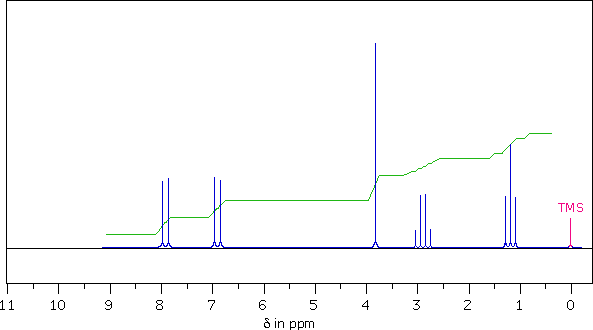
2. What is the multiplicity ( s, d, t, q ) of the highest field signal from this sample?
3. The sample has a singlet at δ = 3.8 ppm. In units of Hertz (Hz) how far is this signal from the TMS signal?
4. What structural feature is suggested by the singlet at δ = 3.8 ppm?
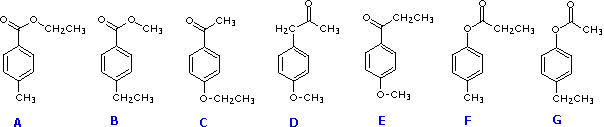
A CH3–C=O B –CH2– C –O–H D –O–CH3 E C–CH3 F C=C–H
5. From multiplet line separations (Js), which of the other signals is coupled to the quartet at δ = 2.9 ppm?
A δ = 1.2 ppm B δ = 3.8 ppm C δ = 6.9 ppm D δ = 7.9 ppm
6. Using the integrator trace and the formula of the sample, assign a whole number ratio to the sample signals as follows:
7.9 ppm signal ; 6.9 ppm signal ; 3.8 ppm signal ; 2.9 ppm signal ; 1.2 ppm signal
The compound's formula, C10H12O2, has less hydrogen than decane (C10H22). The difference of 10 hydrogens suggests the presence of five double bonds and/or rings in the structure. Since a benzene ring has three double bonds and one ring, and the nmr signals at δ = 7.9 and 6.9 ppm are consistent with aryl hydrogen resonances, we conclude that this compound has a benzene ring.
Integration indicates the presence of two methyl groups, a O–CH3 at δ = 3.8 ppm, and a C–CH3 at δ = 1.2 ppm. The former is of course a singlet, but the latter is a triplet (J = 8 Hz) due to coupling with a –CH2– moiety, which in turn is split into a quartet (signal at δ = 2.9 ppm). From the chemical shifts, we conclude there is an ethyl group bonded to an sp2 hybrid carbon atom.
If we examine the structural formulas of the seven candidate compounds, we can eliminate A, C, F & G because they do not have a –O–CH3 group and cannot account for a singlet at δ = 3.8 ppm. Structure D does not have an ethyl group, so we are left with B & E as the most likely source of this spectrum.
It is not a simple job to distinguish B & E by 1Hnmr alone. The simplicity of the aromatic proton region indicates para-disubstitution of the benzene ring. If the two substituents are similar in nature, the ring proton signals would have similar chemical shifts. If the substituents are very different in nature, these signals would also have significantly different chemical shifts. Since the aromatic doublets in this spectrum are separated by 90 Hz (1.0 ppm), we may tentatively select E (oxygen and carbon substituents) over B (two different carbon substituents).
A better means of distinguishing these compounds is by infrared spectroscopy. Ester carbonyl stretching frequencies are usually higher than equivalent ketone frequencies by roughly 25 cm-1. Also the 13Cnmr carbonyl signal from esters (δ = ca. 170 ppm) is usually observed at higher field than ketone carbonyl signals (δ = ca. 200 ppm).
The spectrum is in fact that of E.
1H NMR
13 C NMR
IR

No comments:
Post a Comment