Teriflunomide,
HMR-1726, 1726, A-771726, RS-61980, SU-0020,
(Z)-2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide
108605-62-5, 282716-73-8 (monosodium salt)
C12-H9-F3-N2-O2 270.2091
SPECTROSCOPY DATA ]
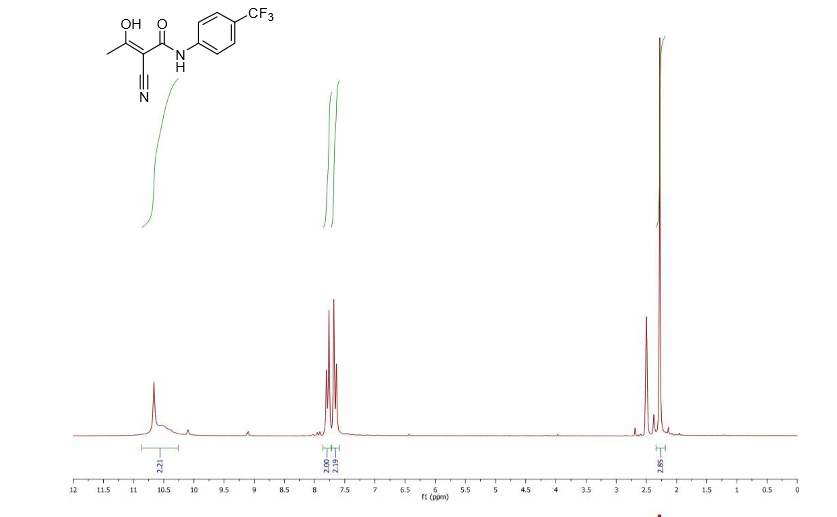
! HNMR (DMSO, 300MHz) :δ 2.24(s, 3H); 5.36(bs, IH); 7.65(d, J=8.7Hz, 2H);
7.76(d, J=8.6Hz, 2H); 10.89(s, IH) ppm.
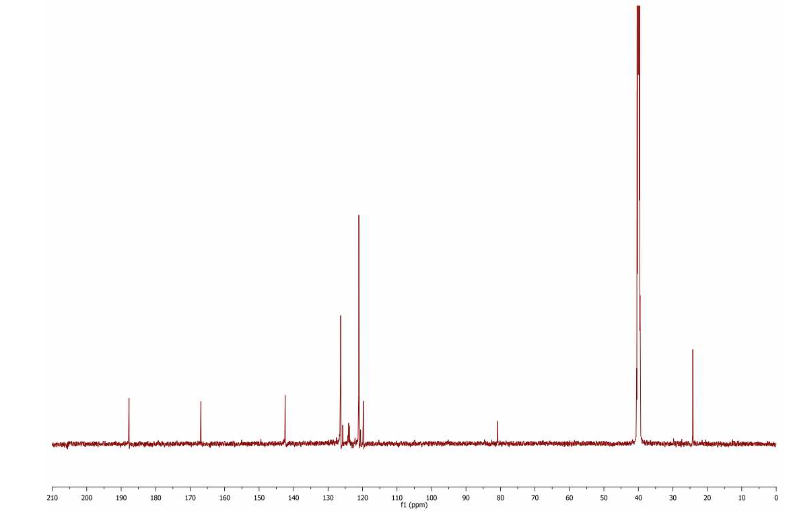
13 CNMR (DMSO, 75MHz) :δ 23.5, 82.1, 118.3, 122.2, 126.5, 126.9, 142.1, 167.4,
187.8 ppm.
MS(FD) : m/e 269(M", 100).
IR : 3305, 2220, 1633, 1596, 1554, 1418, 1405, 1325, 1247, 1114, 1157, 1073, 971,
842, 684 cm-1.
REF EP 2280938 A2UPDATED
TERIFLUNOMIDE SPECTRAL DATA
Teriflunomide,
HMR-1726, 1726, A-771726, RS-61980, SU-0020,
(Z)-2-Cyano-3-hydroxy-N-[4-(trifluoromethyl)phenyl]-2-butenamide
108605-62-5, 282716-73-8 (monosodium salt)
C12-H9-F3-N2-O2 270.2091
SPECTROSCOPY DATA
17= US2011/0105795A1
1H NMR AND 13C NMR
above 13C NMR
! HNMR (DMSO, 300MHz) :δ 2.24(s, 3H); 5.36(bs, IH); 7.65(d, J=8.7Hz, 2H);
7.76(d, J=8.6Hz, 2H); 10.89(s, IH) ppm.
13 CNMR (DMSO, 75MHz) :δ 23.5, 82.1, 118.3, 122.2, 126.5, 126.9, 142.1, 167.4,
187.8 ppm.
MS(FD) : m/e 269(M”, 100).
IR : 3305, 2220, 1633, 1596, 1554, 1418, 1405, 1325, 1247, 1114, 1157, 1073, 971,
842, 684 cm-1.
REF EP 2280938 A2
Example-1 Preparation of Ethyl-2-cyano-3-hydroxy-but-2-enoate (III) [77] Potassium carbonate (73.3 g) was added to the well stirred solution of Ethylcy- anoacetate (50 g) in Dimethylformamide (250 ml) and stirred for 15 minute at ambient temperature. Acetic anhydride (90.25 g) was added drop wise to the above well stirred solution during 2 to 3 hours at ambient temperature. Reaction mixture was stirred at ambient temperature for 15 to 20 hours. Reaction mixture was diluted with water (500 ml) and extracted with dichloromethane (3 xlOO ml). Combined organic layer was washed with saturated sodium carbonate solution (3x100ml). Aqueous carbonate layer was separated and acidified with 50% HCl solution and extracted with dichloromethane (3x100ml). Combined organic layer was washed with brine solution (100 ml), dried over sodium sulfate and evaporated to yield Ethyl 2-cyano-3-hydroxy-but-2-enoate (58 g).
Yield: 84.6% Example-2 Preparation of Teriflunomide (I) [82] Ethyl 2-cyano-3-hydroxybut-2-enoate (III) (50 g) and 4-(trifluoromethyl) aniline (51.9 g) in xylene (1000 ml) was refluxed for 48 hours. The reaction mixture was allowed to cool at room temperature. Separated solid was filtered and washed with xylene (2×100 ml). Solid was dried under vacuum at 700C to yield (62 g) of Teri- flunomide.
Yield: 71.0%
Purity: 99.4%
! HNMR (DMSO, 300MHz) :δ 2.24(s, 3H); 5.36(bs, IH); 7.65(d, J=8.7Hz, 2H);
7.76(d, J=8.6Hz, 2H); 10.89(s, IH) ppm.
13 CNMR (DMSO, 75MHz) :δ 23.5, 82.1, 118.3,
122.2, 126.5,
126.9, 142.1, 167.4,
187.8 ppm.
MS(FD) : m/e 269(M”, 100).
IR : 3305, 2220, 1633, 1596, 1554, 1418, 1405, 1325, 1247, 1114, 1157, 1073, 971,
842, 684 cm-1.
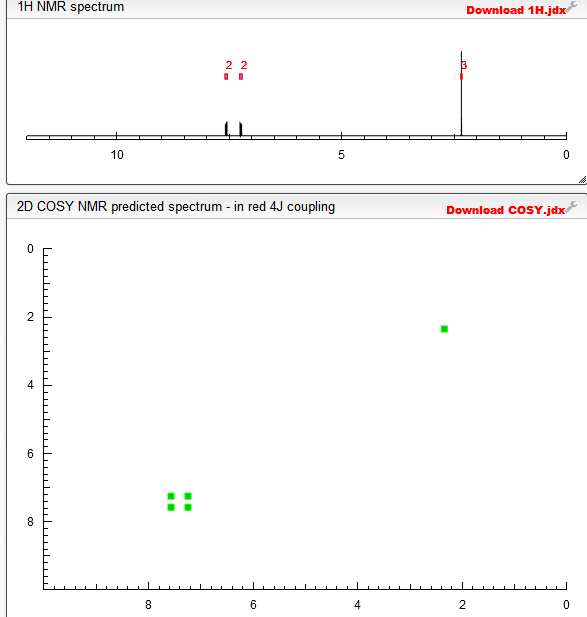
1H NMR PREDICT
COSY
HPLC
HPLC method of analysis:
N-(4′-trifluoromethylphenyI)-5-methylisoxazole-4-carboxamide of formula-2:
Apparatus: A liquid chromatographic system equipped with variable wavelength UV- detector; Column: Cosmicsil APT CI 8, 100 x 4.6 mm, 3 μιη (or) equivalent; Flow rate: 1.5 ml/min; Wavelength: 210 nm; Column Temperature: 25°C; Injection volume: 20 μί; Run time: 40 min; Diluent: Mobile phase; Needle wash: Tetrahydrofuran; Elution: Isocratic; Mobile phase: 5 ml of triethyl amine into a 650 ml of water. Adjusted the pH to 3.4 with dil. Orthophosphoric acid and filter this solution through 0.22 μπι nylon membrane filter paper and sonicate to degas it. (Z)-2-cyano-3-hydroxy-but-2-enoicacid-(4-trifluoromethyl phenyl)-amide compound of formula- 1:
Apparatus: A liquid chromatographic system equipped with variable wavelength UV- detector; Column: Kromasil 100 C18, 250 x 4.6 mm, 5 μηι (or) equivalent; Flow rate: 1.0 ml/min; Wavelength: 250 nm; Column Temperature: 35°C; Injection volume: 5 μί; Run time: 37 min; Diluent: 0.01 M dipotassium hydrogen orthophosphate in 1000 ml of water; Elution: Gradient; Mobile phase-A: Buffer (100%); Mobile phase-B: Acetonitrile : Buffer (70:30 v/v); Buffer: 1 ml of ortho phosphoric acid into a 1000 ml of water and 3.0 grams of 1 -octane sulfonic acid sodium salt anhydrous. Adjust pH to 6.0 with potassium hydroxide solution and filtered through 0.22μηι Nylon membrane filter paper and sonicate to degas it……..http://www.google.com/patents/WO2015029063A2?cl=en
| WO2009147624A2* | 3 Jun 2009 | 10 Dec 2009 | Alembic Limited | A process for preparing teriflunomide |
| WO2011004282A2* | 22 Jun 2010 | 13 Jan 2011 | Alembic Limited | Novel polymorphic form of teriflunomide salts |
| US5494911 | 24 Oct 1990 | 27 Feb 1996 | Hoechst Aktiengesellschaft | Isoxazole-4-carboxamides and hydroxyalkylidenecyanoacetamides, pharmaceuticals containing these compounds and their use |
| US5679709 | 7 Jun 1995 | 21 Oct 1997 | Hoechst Aktiengesellschaft | N-(4-trifluoromethylphenyl)-2-cyano-3-hydroxycrotonamide or salts, used for reduction of b-cell produced self-antibodies |
| US5990141 | 6 Jan 1995 | 23 Nov 1999 | Sugen Inc. | Administering 5-methyl-isoxazole-4-carboxylic acid-n-(4-trifluoromethyl)anilide or 2-cyano-3-hydroxy-n-(4-trifluoro-methyl)phenyl-2-butenamide; antitumor,-carcinogenic and proliferative agents; kinase inhibitors |
riga latvia
..........

No comments:
Post a Comment