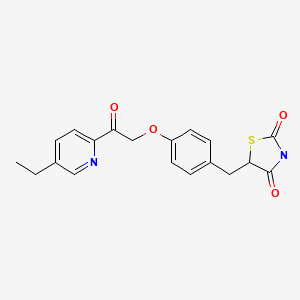
MITOGLITAZONE
MSDC-0160; CAY 10415; 146062-49-9
5-[4-[2-(5-Ethylpyridin-2-yl)-2-oxoethoxy]benzyl]thiazolidine-2,4-dione
5-[[4-[2-(5-ethylpyridin-2-yl)-2-oxoethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
5-(4-(2-(5-cthylpyridin-2-yl)- 2-oxoethoxy)benzyl)-1,3 -thiazolidiiie-2,4-dione
Pfizer, INNOVATOR phase 2
MSD-9
MSDC-0160
PNU-91325
U-91325
MSDC-0160
PNU-91325
U-91325
J. Med. Chem., 1996, 39 (26), pp 5053–5063
DOI: 10.1021/jm9605694
Pioglitazone (5-(4-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione, 2) is a prototypical antidiabetic thiazolidinedione that had been evaluated for possible clinical development. Metabolites 6−9 have been identified after dosing of rats and dogs. Ketone 10has not yet been identified as a metabolite but has been added to the list as a putative metabolite by analogy to alcohol 6and ketone 7. We have developed improved syntheses of pioglitazone (2) metabolites 6−9 and the putative metabolite ketone 10. These entities have been compared in the KKAy mouse model of human type-II diabetes to pioglitazone (2). Ketone 10 has proven to be the most potent of these thiazolidinediones in this in vivo assay. When 6−10 were compared in vitro in the 3T3-L1 cell line to 2, for their ability to augment insulin-stimulated lipogenesis, 10 was again the most potent compound with 6, 7, and 9roughly equivalent to 2. These data suggest that metabolites 6, 7, and 9 are likely to contribute to the pharmacological activity of pioglitazone (2), as had been previously reported for ciglitazone (1).
5-((4-(2-(5-Ethyl-2-pyridyl)-1-oxoethoxy)phenyl)methyl)-2,4-thiazolidinedione (10). MITOGLITAZONE
free flowing white powdery solid
(mp 146−147 °C):
TLC (Merck; MeOH−CH2Cl2, 5:95, UV(+)) Rf = 0.21;
1H-NMR (CDCl3) δ 8.95 (brs, 1), 8.52 (d, J = 2.0 Hz, 1), 8.02 (d, J = 8.0 Hz, 1),
7.70 (dd, J = 8.0, 2.0 Hz, 1), 7.16 (d, J = 8.7 Hz, 2), 6.94 (d, J = 8.7 Hz, 2),
5.62 (s, 2), 4.49 (dd, J = 9.7, 3.8 Hz, 1), 3.47 (dd, J = 14.2, 3.8 Hz, 1),
3.08 (dd, J = 14.2, 9.7 Hz, 1), 2.76 (q, J = 7.6 Hz, 2), 1.31 (t, J = 7.6 Hz, 3);
13C-NMR (CDCl3) δ 194.7, 174.1, 170.4, 157.7, 149.7, 148.9, 144.7, 136.3, 130.3, 128.4, 121.9, 115.2, 70.5, 53.7, 37.8, 26.4, 15.0;
EI/MS (70 eV) 370 (M+, 19.4), 341 (6.9), 254 (20.6), 148 (base).
Anal. (C19H18N2O4S) C, H, N, S.
……………………………….
1H NMR PREDICTION

No comments:
Post a Comment