Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
J. Org. Chem., 2013, 78 (14), pp 7048–7057
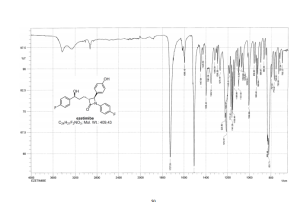
Ezetimibe (1)
ezetimibe 1 (1.08 g, 80%) as a white solid.
Mp 164–166 °C [lit.(11) 155–157 °C];
99% ee;
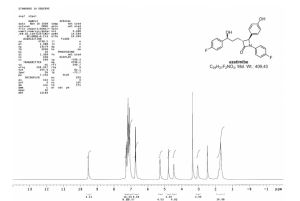
1H NMR (600 MHz, DMSO-d6) δ 9.49 (1H, s), 7.28–7.24 (2H, m), 7.19–7.16 (4H, m), 7.11–7.07 (4H, m), 6.75–6.71 (2H, m), 5.25 (1H, d, J 4.3 Hz), 4.77 (1H, d, J 2.2 Hz), 4.49–4.59 (1H, m), 3.07–3.04 (1H, m) 1.84–1.66 (4H, m);
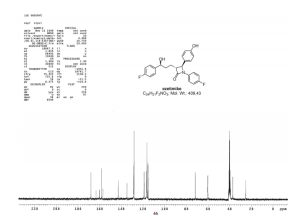
13C NMR (150 MHz, CDCl3) δ 167.8, 162.3, and 160.7 (d, JC–F 240.3 Hz), 159.3, 157.9, 157.7, 142.5, 134.4, 128.7, 128.3, 128.0, 127.9, 118.7, and 118.6 (d, JC–F 8.1 Hz), 116.3, 116.2, 115.2, and 115.0 (d, JC–F 20.7 Hz), 71.5, 60.0, 59.9, 36.8, 24.9;
HRMS (EI, TOF) m/z calcd for C24H21F2NO3 [M] 409.1489 found 409.1478.
Anal. Calcd for C24H21F2NO3: C 70.41, H 5.17, F 9.28, N 3.42. Found: C 70.46, H 5.23, F 9.24, N 3.34.
Anal. Calcd for C24H21F2NO3: C 70.41, H 5.17, F 9.28, N 3.42. Found: C 70.46, H 5.23, F 9.24, N 3.34.
(3S,4S)-4-(4-(Benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3′-hydroxypropyl)azetidin-2-one (20)
Method 1
To a cooled (0 °C) solution of lactone 19 (2.0 g, 4 mmol) in 160 mL of dry diethyl ether was added 12 mL of 1 M solution of t-BuMgCl in diethyl ether. After 2 h, 30 mL of aq NH4Cl was added. The aqueous layer was extracted with ether (160 mL), the organic layer was washed with satd NaHCO3 (50 mL) and dried (MgSO4), and the solvent was removed under reduced pressure. Crude product 20 (1.64 g, 82%) obtained as a yellowish solid was used in the next step without further purification. An analytic sample was obtained by chromatography on silica gel (hexanes/ethyl acetate 7:3). Mp 130–133 °C [lit.(11) 132–134 °C]; [α]20D −42.2 (c 1.2, CHCl3); 1H NMR (600 MHz, CDCl3) δ 7.42–7.20 (11H, m), 7.02–6.90 (6H, m), 5,04 (2H, s), 4.72–4.68 (1H, m), 4.55 (1H, d J 2.2 Hz), 3.07 (1H, dt J 7.1, 2.2 Hz), 2.05–1.93 (3H, m) 1.89–1.82 (2H, m); 13C NMR (150 MHz, CDCl3) δ 167.6, 163.0, and 161.4 (d, JC–F 244.2 Hz), 159.8 and 158.1 (d, JC–F 241.8 Hz), 159.0, 140.0, 139.9, 136.6, 133.9, and 133.8 (d, JC–F 2.9 Hz), 129.6, 128.6, 128.1, 127.5, 127.4 and 127.4, (d, JC–F 8.0 Hz), 127.2, 118.4, 118.3, 115.8, 115.8, and 115.7 (d, JC–F 22.0 Hz), 115.5, 115.4, and 115.3 (d, JC–F 21.3 Hz), 73.3, 70.1, 61.1, 60.3, 36.5, 25.0; HRMS (ESI, TOF) m/z calcd for C31H27F2NO3Na [M + Na]+ 522.1851, found 522.1862; IR (KBr) v 3441, 1743, 1609, 1510 cm–1. Anal. Calcd for C31H27F2NO3: C 74.53, H 5.45, N 2.80, F 7.61. Found: C 74.40, H 5.53, N 2.74, F 7.56.
Org. Process Res. Dev., 2009, 13 (5), pp 907–910
DOI: 10.1021/op900039z
Preparation of 1-(4-Fluorophenyl)-3-(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone 1 (Ezetimibe)
of compound 1. 1H NMR (300 MHz, DMSO-d6, δ) 1.72−1.84 (m, 4H), 3.08 (m, 1H), 4.45 (m, 1H), 4.8 (d, 1H, J = 2.0 Hz), 5.25 (d, 1H, J = 4.8), 6.75 (d, 2H, J = 8.4 Hz), 7.05−7.4 (m, 10H, Ar), 9.48 (s, 1H); IR: 3270.0, 2918, 1862, 1718.4, 1510 cm−1. MS: m/z 409.2 (M+). Anal. Calcd for C15H17NO5: C, 70.41; H, 5.17; N, 3.42. Found: C, 70.38; H, 5.27; N, 3.34.
Preparation of (3R,4S)-1-(4-Fluorophenyl)-3-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone 10
compound 9 as a white solid. 1H NMR (200 MHz, DMSO-d6, δ) 1.6−1.9 (m, 4H), 2.0−2.2 (bs, 1H), 3.0−3.2 (m, 1H), 4.4−4.6 (m, 1H), 4.74 (m, 1H), 5.05 (s, 2H), 6.95−7.9 (m, 17H, Ar); IR: 3492, 2922, 2852, 1719 cm−1; MS: m/z 499.3 (M+).
....
- Table 2.1H and 13C NMR assignments for Eze-1 and desfluoro Eze-1.
Positiona 1H–δ ppm
13C–δ ppm (DEPT)
Eze-1b Desfluoro Eze-1b Eze-1b Desfluoro Eze-1b 1 10.15 (br, OH) 10.13 (br, OH) – – 2 – – 161.3 (C) 161.3 (C) 3 6.87 (d, J=8.5 Hz, 2H) 6.87 (dd, J=8.4, 1.8 Hz, 2H) 116.3 (2CH) 116.3 (2CH) 4 7.74 (d, J=8.5 Hz, 2H) 7.75 (dd, J=8.4, 1.8 Hz, 2H) 131.4 (2CH) 131.4 (2CH) 5 – – 128.1 (C) 128.2 (C) 6 8.43 (s, 1H) 8.43 (s, 1H) 160.8 (CH) 160.8 (CH) 7 – – 149.0 (d, 4J=2.6 Hz, C) 152.7 (C) 8 7.15–7.26 (m, 4H) 7.36 (dd, J=8.1, 7.5 Hz, 2H) 123.3 (d, 3J=8.4 Hz, 2CH) 121.6 (2CH) 9 7.17 (d, J=7.8 Hz, 2H) 116.5 (d, 2J=22 Hz, 2CH) 129.8 (2CH) 10 – 7.18 (t, J=6.3 Hz, 1H) 160.8 (d, 1J=242 Hz, C) 126.0 (CH) - Assignments: s: singlet; d: doublet; t: triplet; m: multiplet; br: broad singlet. Mean values used for coupled signals.
-
- aNumbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A.
- bSolvent is DMSO-d6.
R-Enantiomer in Ezetimibe
R-Enantiomer in Ezetimibe
Isolation and Characterization of R-Enantiomer in Ezetimibe
www.scirp.org/journal/PaperDownload.aspx?paperID=36901
by K Chimalakonda - 2013 - Related articles
HPLC1H and 13C NMR. The purity of isolated R-Isomer is about 98%. Keywords: Isolation; Characterization; (R)-Isomer; Ezetimibe; Supercritical Fluid ...
1H NMR VALUES FOR R ENANTIOMER
13C NMR OF R ENANTIOMER
13C NMR VALUES OF R ENANTIOMER
IR OF R ENANTIOMER
Ezetimibe for reference
| Systematic (IUPAC) name | |
|---|---|
| (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one | |
| Clinical data | |
| Trade names | Zetia |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a603015 |
| Legal status | |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 35–65% |
| Protein binding | >90% |
| Metabolism | Intestinal wall, hepatic |
| Half-life | 19–30 hours |
| Excretion | Renal 11%, faecal 78% |
| Identifiers | |
| CAS number | 163222-33-1 |
| ATC code | C10AX09 |
| PubChem | CID 150311 |
| DrugBank | DB00973 |
| ChemSpider | 132493 |
| UNII | EOR26LQQ24 |
| KEGG | D01966 |
| ChEBI | CHEBI:49040 |
| ChEMBL | CHEMBL1138 |
| Chemical data | |
| Formula | C24H21F2NO3 |
| Molecular mass | 409.4 g·mol−1 |
| Physical data | |
| Melting point | 164 to 166 °C (327 to 331 °F) |
| | |
1H NMR OF R ENANTIOMER PREDICTED
cosy
.
Ezetimibe has the chemical name
1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone
(hereinafter referred to by its adopted name “ezetimibe”) and is
structurally represented by Formula I.
Ezetimibe is in a class of lipid lowering compounds that selectively
inhibit the intestinal absorption of cholesterol and related
phytosterols. It is commercially available in products sold using the
trademark ZETIA as a tablet for oral administration containing 10 mg of
ezetimibe, and in combination products with simvastatin using the
trademark VYTORIN.
U.S. Pat. No. 6,096,883 discloses generically and specifically ezetimibe
and its related compounds along with their pharmaceutical compositions.
The patent also describes a process for the preparation of ezetimibe.
The process described in the patent involves the use of
methyl-4-(chloroformyl) butyrate and also involves isolation of the
compound
(3R,4S)-1-(4-fluorophenyl)-3-[3-(chloroformyl)-3-oxo-propyl]-4-(4-benzyloxyphenyl)-2-azetidinone
as an intermediate. Chlorinated compounds are unstable and difficult to
handle in large scale productions. The process described in the patent
also involves the purification of intermediates using column
chromatography, thus making the process difficult to be scaled up.
Processes for preparation of ezetimibe and its intermediates have also
been described in U.S. Pat. Nos. 6,207,822, 5,856,473, 5,739,321, and
5,886,171, International Application Publication No. WO 2006/050634, and
in Journal of Medicinal Chemistry 1998, 41, 973-980, Journal of Organic Chemistry 1999, 64, 3714-3718, and Tetrahedron Letters, 44(4), 801-804.
http://www.google.com/patents/US20070049748
EXAMPLE 10 PREPARATION OF 1-(4-FLUOROPHENYL)-3(R)-[3-(4-FLUOROPHENYL)-3(S)-HYDROXYPROPYL]-4(S)-(4-HYDROXYPHENYL)-2-AZETIDINONE (FORMULA I)
50 g of
(3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3(s)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone
and 475 ml of methanol were taken into a round bottom flask. A mixture
of 15 g of 5% palladium on carbon and 25 ml of water was added to it.
The reaction mass was flushed with hydrogen gas and a hydrogen pressure
of 3 to 5 kg/cm2 was applied. The reaction mass was stirred for 3 hours.
Reaction completion was checked using thin layer chromatography. After
the reaction was completed, the pressure was released and the reaction
mass was filtered through perlite. The filter bed was washed with 100 ml
of methanol. The filtrate was distilled completely at 70° C., and 400
ml of isopropanol was added to it. The reaction mass was heated to 45°
C. and maintained for 10 minutes. The reaction mass was then allowed to
cool to 28° C. 400 ml of water was added to the reaction mass and
stirred for 1 hour, 20 minutes. The separated compound was filtered and
washed with 100 ml of water. The wet cake was taken into another round
bottom flask and 500 ml of chlorobenzene and 40 ml of methanol were
added to it. The reaction mass was heated to 65° C. and maintained for
15 minutes. 25 ml of water was added to the reaction mass and stirred
for 2 hours. The separated compound was filtered and washed with 100 ml
of chlorobenzene. The wet cake was taken into another round bottom flask
and 375 ml of chlorobenzene, and 30 ml of methanol were added to it.
The reaction mass was heated to 62° C. and maintained for 10 minutes.
The reaction mass was then cooled to 28° C. and 20 ml of water was added
to it. The reaction mass was stirred for 20 minutes and then filtered
and washed with 100 ml of chlorobenzene. The wet cake was taken into
another round bottom flask and 400 ml of isopropanol was added to it.
The reaction mass was heated to 46° C. and maintained for 15 minutes.
800 ml of water was added to the reaction mass at 45 to 46° C. and
stirred for one hour. The separated solid was filtered and washed with
water. The process of recrystallization in a combination of isopropanol
and water was repeated and the obtained compound was dried at 70° C. for
5 hours to get 19.8 g of the title compound. (Yield 49.2%)
Purity by HPLC: 99.68%.
EXAMPLE 11 PURIFICATION OF 1-(4-FLUOROPHENYL)-3(R)-[3-(4-FLUOROPHENYL)-3(S)-HYDROXYPROPYL]-4(S)-(4-HYDROXYPHENYL)-2-AZETIDINONE (FORMULA I)
15.0 g of ezetimibe obtained above and 120 ml of isopropanol were taken
into a round bottom flask and the reaction mass was heated to 48° C. The
reaction mass was filtered through a perlite bed in the hot condition
to make the solution particle free. The filtrate was taken into another
round bottom flask and heated to 47° C. 240 ml of water was added at 47°
C. After completion of the addition, the reaction mass was maintained
at 47° C. for 1 hour. The separated solid was filtered and washed with
30 ml of water. The wet compound was dried at 70° C. for 8 hours to get
13.4 g of the title compound. (Yield 89%)
Purity by HPLC: 99.92.
benzyl ezetimibe impurity: less than 0.0003 area-%,
benzyl ezetimibe diol impurity: 0.004 area-%,
lactam cleaved alcohol impurity: 0.003 area-%,
lactam cleaved acid impurity: 0.01 area-%,
ezetimibe diol impurity: less than 0.0007 area-%.
Residual solvent content by gas chromatography:
Isopropyl alcohol: 1454 ppm
All other solvents: Less than 100 ppm.
| WO1997045406A1 * | May 28, 1997 | Dec 4, 1997 | Schering Corp | 3-hydroxy gamma-lactone based enantioselective synthesis of azetidinones |
| WO2004099132A2 | May 5, 2004 | Nov 18, 2004 | Ram Chander Aryan | Process for the preparation of trans-isomers of diphenylazetidinone derivatives |
| WO2008032338A2 * | Sep 10, 2007 | Mar 20, 2008 | Reddy Maramreddy Sahadeva | Improved process for the preparation of ezetimibe and its intermediates |
| EP0720599A1 | Sep 14, 1994 | Jul 10, 1996 | Schering Corporation | Hydroxy-substituted azetidinone compounds useful as hypocholesterolemic agents |
| US20070049748 | Aug 25, 2006 | Mar 1, 2007 | Uppala Venkata Bhaskara R | Preparation of ezetim |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US7470678 | Jul 1, 2003 | Dec 30, 2008 | Astrazeneca Ab | Diphenylazetidinone derivatives for treating disorders of the lipid metabolism |
| US7842684 | Apr 25, 2007 | Nov 30, 2010 | Astrazeneca Ab | Diphenylazetidinone derivatives possessing cholesterol absorption inhibitor activity |
| US7863265 | Jun 19, 2006 | Jan 4, 2011 | Astrazeneca Ab | N-{[4-((2R,3R)-1-(4-fluorophenyl)-3-{[(2R or S)-2-(4-fluorophenyl)-2-hydroxyethyl]thio}-4-oxoazetidin-2-yl)phenoxy]acetyl}glycyl-D-lysine, used as anticholesterol agents |
| US7871998 | Dec 21, 2004 | Jan 18, 2011 | Astrazeneca Ab | Diphenylazetidinone derivatives possessing cholesterol absorption inhibitory activity |
| US7893048 | Jun 21, 2006 | Feb 22, 2011 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| US7906502 | Jun 21, 2006 | Mar 15, 2011 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| US8013150 * | Feb 17, 2006 | Sep 6, 2011 | Msn Laboratories Ltd. | Process for the preparation of ezetimibe |
| US8383810 | Dec 12, 2011 | Feb 26, 2013 | Merck Sharp & Dohme Corp. | Process for the synthesis of azetidinones |
| US20110130378 * | May 26, 2009 | Jun 2, 2011 | Lek Pharmaceuticals D.D. | Ezetimibe process and composition |
| US20110183956 * | Jul 29, 2009 | Jul 28, 2011 | Janez Mravljak | Process for the synthesis of ezetimibe and intermediates useful therefor |
| EP2128133A1 | May 26, 2008 | Dec 2, 2009 | Lek Pharmaceuticals D.D. | Ezetimibe process and composition |
| WO2008096372A2 * | Feb 6, 2008 | Aug 14, 2008 | Pranav Gupta | Process for preparing highly pure ezetimibe using novel intermediates |
| WO2009150038A1 | May 26, 2009 | Dec 17, 2009 | Lek Pharmaceuticals D.D. | Process for the preparation of ezetimibe and composition containing it |
| WO2009157019A2 * | Jun 23, 2009 | Dec 30, 2009 | Ind-Swift Laboratories Limited | Process for preparing ezetimibe using novel allyl intermediates |
| WO2005021497A2 * | Aug 27, 2004 | Mar 10, 2005 | Eduardo J Martinez | Tethered dimers and trimers of 1,4-diphenylazetidn-2-ones |
| WO2006127893A2 * | May 25, 2006 | Nov 30, 2006 | Microbia Inc | Processes for production of 4-(biphenylyl)azetidin-2-one phosphonic acids |
| WO2008096372A2 * | Feb 6, 2008 | Aug 14, 2008 | Pranav Gupta | Process for preparing highly pure ezetimibe using novel intermediates |
| US20070049748 * | Aug 25, 2006 | Mar 1, 2007 | Uppala Venkata Bhaskara R | Preparation of ezetimibe |
Ziguinchor
Town in Senegal
Ziguinchor
is the capital of the Ziguinchor Region, and the chief town of the
Casamance area of Senegal, lying at the mouth of the Casamance River. It
has a population of over 230,000. Wikipedia



/////



















.png)
.png)
.png)
.png)



Nice Blog...
ReplyDeleteThanks For Sharing These Blog With Us...
Ezetimibe Manufacturers