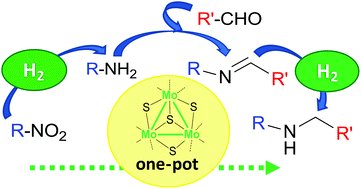
Secondary amines are selectively obtained from low value starting materials using hydrogen and a non-noble metal-based catalyst. The reductive amination of aldehydes from nitroarenes or nitroalkanes is efficiently catalyzed by a well-defined diamino molybdenum sulfide cluster in a one-pot homogeneous reaction. The integrity of the molecular cluster catalyst is preserved along the process.
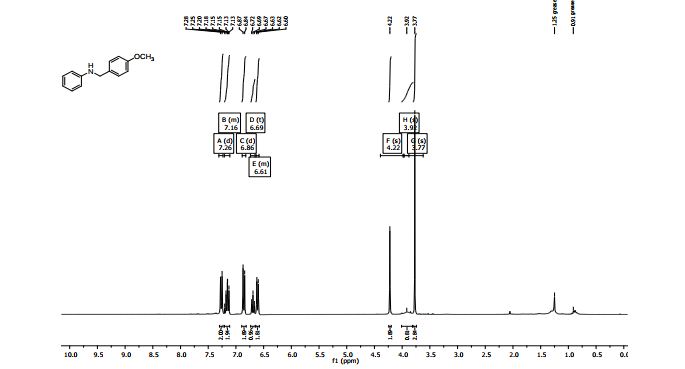
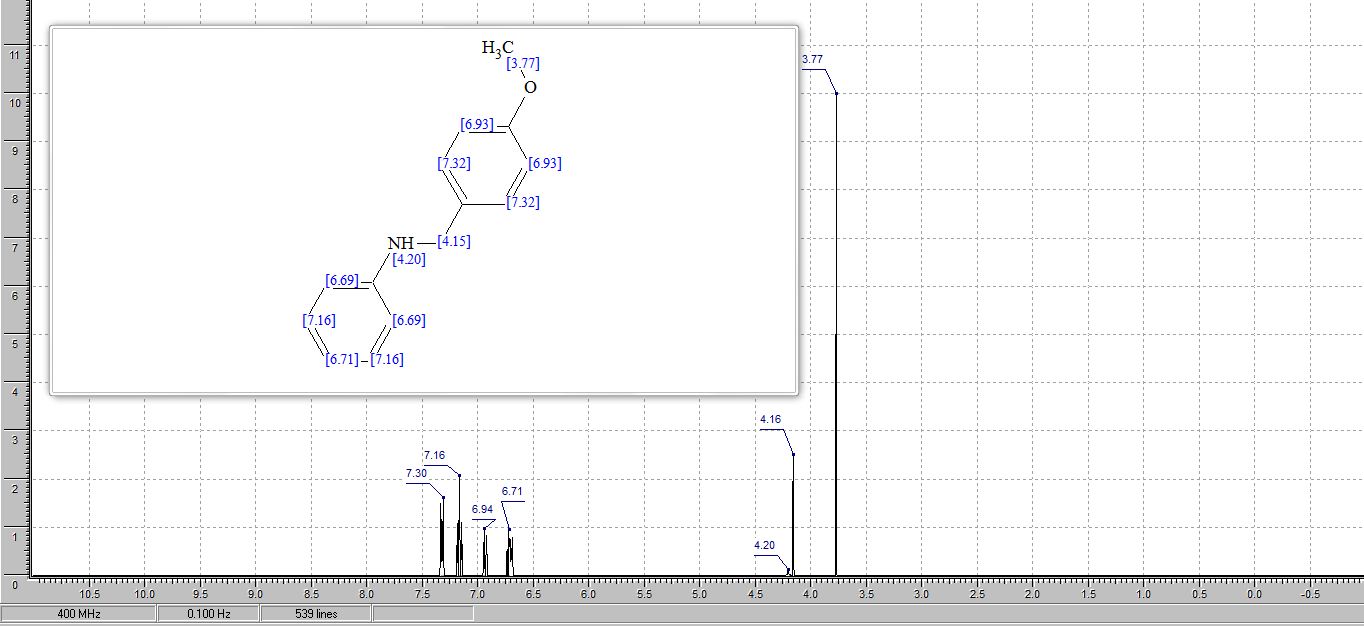
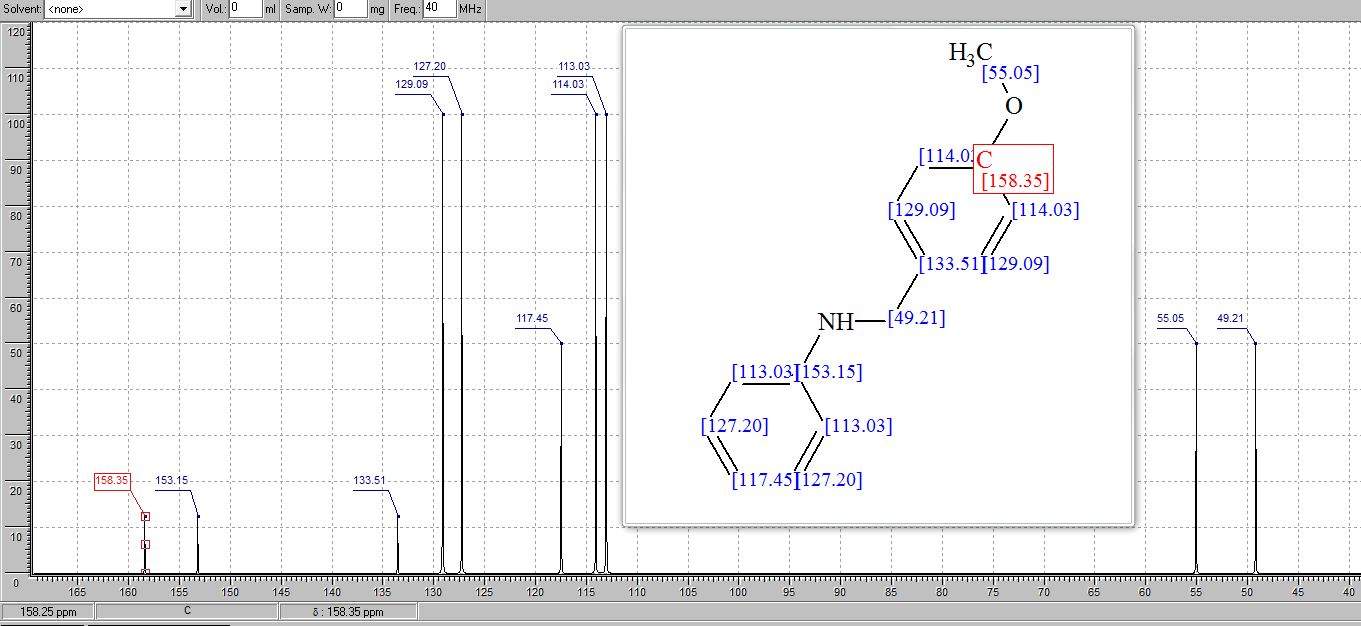
N-(4’-Methoxybenzyl)aniline3 :
1H NMR (300 MHz, CDCl3) δ 7.26 (d, J = 8.6 Hz, 2H), 7.21 – 7.11 (m, 2H), 6.86 (d, J = 8.7 Hz, 2H), 6.69 (t, J = 7.3 Hz, 1H), 6.64 – 6.58 (m, 1H), 4.22 (s, 2H), 3.92 (br s, 1H), 3.77 (s, 3H);
13C NMR (75 MHz, CDCl3) δ 158.96, 148.32, 131.53, 129.35, 128.90, 117.59, 114.13, 112.94, 55.39, 47.89;
MS (EI): m/z (rel. Int) 213.
1H AND 13C NMR PREDICTIONS









 Open Access
Open Access