Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC02261A, Communication
DOI: 10.1039/C7GC02261A, Communication
Shawn Parisien-Collette, Corentin Cruche, Xavier Abel-Snape, Shawn K. Collins
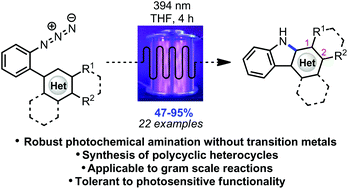
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor.
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor.
Photochemical intramolecular amination for the synthesis of heterocycles
Abstract
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor. The experimental set-up is tolerant to UV-sensitive functional groups while affording diverse carbazoles, as well as an indole and pyrrole framework, in short reaction times. The photochemical method is presumed to progress through a mechanism differing from the other methods of azide activation involving transition metal catalysis.

Methyl 9H-carbazole-2-carboxylate (9): Following the Photodecomposition Procedure A, starting from Methyl 2’-azido-[1,1’-biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (24.3 mg, 72 % yield). Following the Photodecomposition Procedure B, starting from Methyl 2’-azido-[1,1’- biphenyl]-4-carboxylate, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a white solid (27.7 mg, 82 % yield). NMR data was in accordance with what was previously reported.16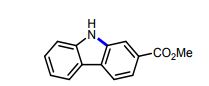
16 Takamatsu, K.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2014, 16, 2892-2895
NEXT..............
4-Isopropyl-9H-carbazole (14): Following the Photodecomposition Procedure A, starting from 2-azido-2’-isopropyl-1,1’-biphenyl, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.6 mg, 53 % yield). Following the Photodecomposition Procedure B, starting from 2-azido-2’-isopropyl-1,1’-biphenyl and using ethyl acetate as the solvant, the crude mixture was purified by silica gel column chromatography (100 % hexanes → 10 % ethyl acetate in hexanes), to afford the desired product as a yellow solid (16.0 mg, 51 % yield).
1H NMR (400 MHz, DMSO-d6) δ = 11.29 (s, 1H), 8.11 (d, J = 8.1 Hz, 1H), 7.50 (d, J = 8.1 Hz, 1H), 7.39-7.31 (m, 3H), 7.19- 7.15 (m, 1H), 7.08-7.03 (m, 1H), 3.92-3.82 (m, 1H), 1.41 (d, J = 6.8 Hz, 6H);
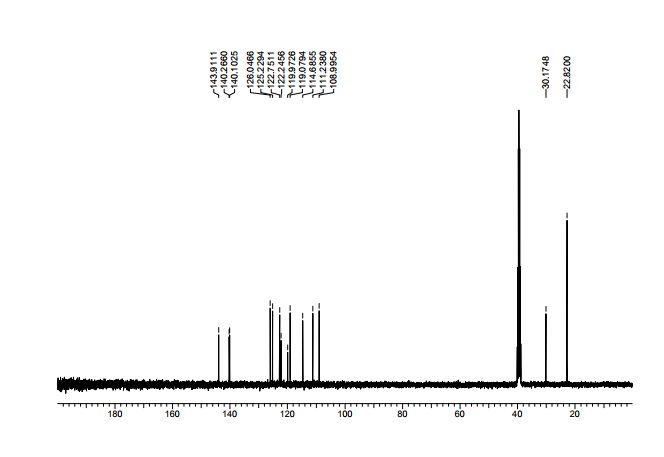
13C NMR (100 MHz, DMSO-d6) δ = 143.9, 140.3, 140.1, 126.0, 125.2, 122.8, 122.2, 119.9, 119.1, 114.9, 111.2, 108.9, 30.2, 22.8 (2C);
HRMS (ESI) m/z calculated for C15H15N [M-H]- 208.1130; found 208.1126.

No comments:
Post a Comment