

Ukraine

Abstract
The synthesis of monocyclic, spirocyclic and fused bicyclic secondary amines bearing a gem-difluorocyclopropane moiety via difluorocyclopropanation of unsaturated N-Boc derivatives using the trifluoromethyl(trimethyl)silane/sodium iodide [CF3SiMe3-NaI] system is described. The relative order of the substrate reactivity is established. It is shown that for the reactive alkenes the standard reaction conditions can be used, whereas for the substrates with low reactivity, slow addition of the Ruppert–Prakash reagent is necessary.

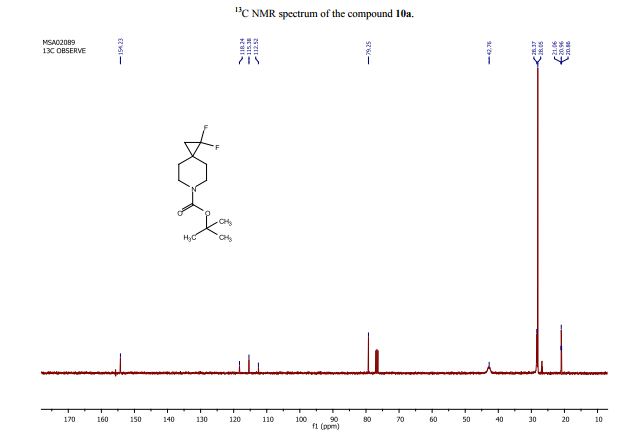
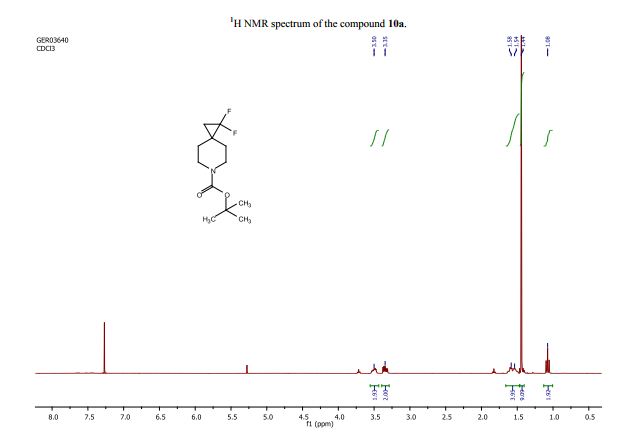
tert-Butyl 1,1-difluoro-6-azaspiro[2.5]octane-6-carboxylate (10a):
Yield: 66.7 g (91%) (Method A); off-white crystalline powder: mp 46–48 8C;
1H NMR (CDCl3 , 400 MHz): d= 3.57–3.42 (m, 2H), 3.40–3.27 (m, 2H), 1.66–1.47 (m, 4H), 1.44 (s, J=2.3 Hz, 9H), 1.08 (t, J=8.3 Hz, 2H);
13C NMR (CDCl3, 101 MHz): d=154.2, 115.4 (t, J=288.1 Hz), 79.3, 42.8, 28.4, 28.1, 26.8 (t, J=10.0 Hz), 21.0 (t, J=10.1 Hz);
19F NMR (CDCl3 , 376 MHz): d=@140.6;
MS (EI): m/z= 247 (M+ ), 192 (M+@t-Bu), 174 (M+@t-BuO), 147 (M+@Boc), 127 (M+@Boc@HF);
Anal. calcd. for C12H19F2NO2 : C 58.29, H 7.74, N 5.66; found: C 58.49, H 8.02, N 5.30.







No comments:
Post a Comment