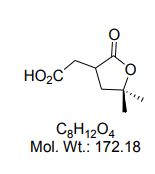
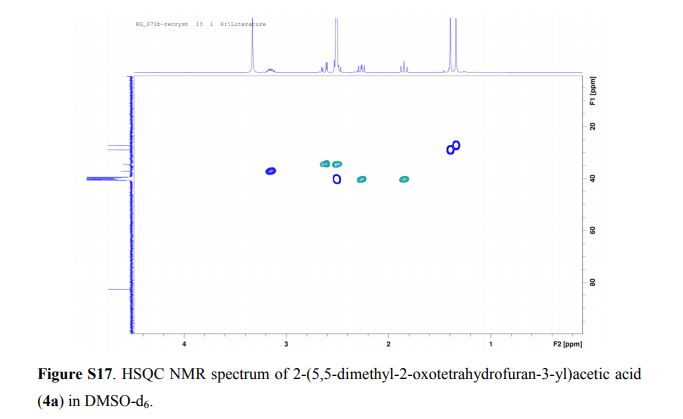
(5,5-dimethyl-2-oxotetrahydrofuran-3-yl)acetic acid
2-(5,5-Dimethyl-2-oxotetrahydrofuran-3-yl)acetic acid
| 2-carboxymethyl-4-methyl-4-pentanolide, |
Cas 412298-86-3
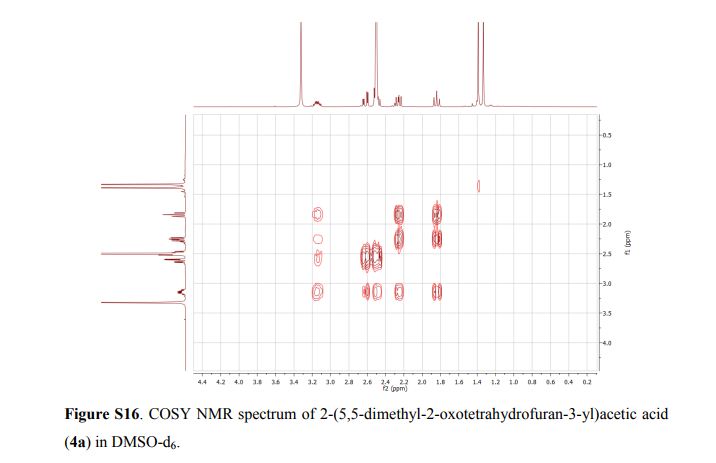
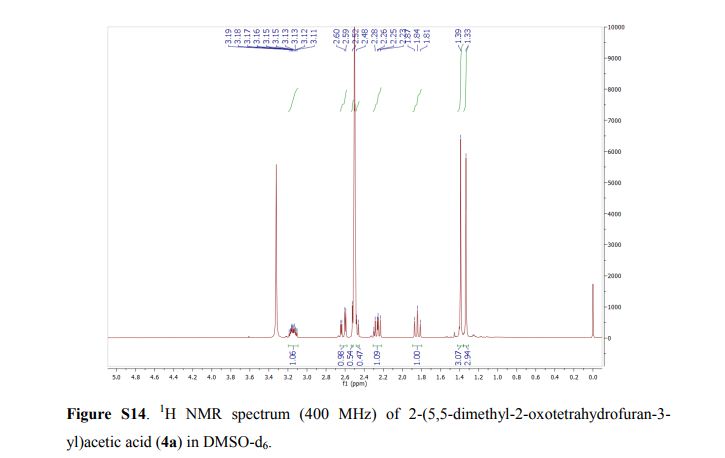
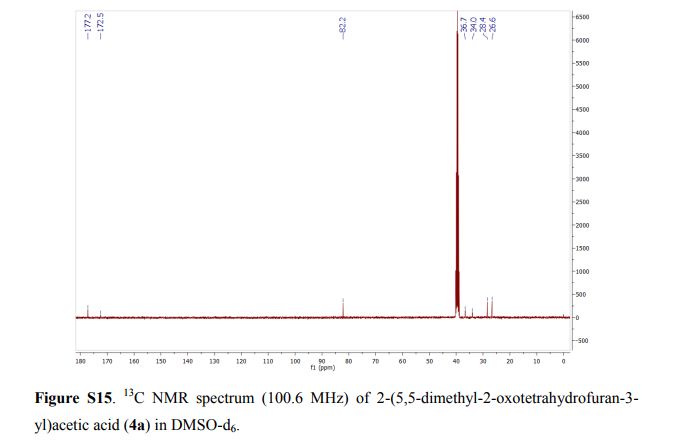
2-(5,5-Dimethyl-2-oxotetrahydrofuran-3-yl)acetic acid (4a).
1H NMR (DMSO-d6, 400 MHz): δ = 3.20 – 3.10 (m, 1H), 2.62 (dd, J = 17.1, 4.4 Hz, 1H), 2.54 – 2.45 (m, 1H), 2.31 – 2.21 (m, 1H), 1.84 (t, J = 12.0 Hz, 1H), 1.39 (s, 3H), 1.33 (s, 3H) ppm.
13C NMR (DMSO-d6, 100.6 MHz): δ = 177.2, 172.5, 82.2, 39.8, 36.7, 34.0, 28.4, 26.6 ppm.
The 1H NMR data did not match those reported in the literature. S7 IR (neat): νmax = 2980, 2935, 1728, 1707 cm- 1 .
MP: 132.1-133.8 °C (lit.S8 137-140 °C).
[S7] Kochikyan, T. V.; Arutyunyan, E. V.; Arutyunyan, V. S.; Avetisyan, A. A. Russ. J. Org. Chem. 2002, 38, 390–393.
[S8] Phillips, D. D.; Johnson, W. A. J. Am. Chem. Soc. 1955, 77, 5977–5982.
ESI HRMS m/z C8H11O4 - [M-H]- : calcd 171.0652. Found 171.0653.
Continuous-Flow Preparation of γ-Butyrolactone Scaffolds from Renewable Fumaric and Itaconic Acids under Photosensitized Conditions
† Center for Integrated Technology and Organic Synthesis, Department of Chemistry, University of Liège, B-4000 Liège (Sart Tilman), Belgium
‡ Corning Reactor Technologies, Corning SAS, 7 bis Avenue de Valvins, CS 70156 Samois sur Seine, 77215 Avon Cedex, France
§ XStruct, Department of Chemistry, Ghent University, Krijgslaan 281-S3, B-9000 Ghent, Belgium
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00314
*E-mail: jc.monbaliu@ulg.ac.be.
Abstract
The method and results described herein concern the photosensitized addition of various alcohols to renewable platform fumaric and itaconic acids under scalable continuous-flow conditions in glass micro- and mesofluidic reactors. Alcohols were used both as reagents and as solvents, thus contributing to a reduced environmental footprint. Process parameters such as the temperature, light intensity, and the nature as well as amount of the photosensitizer were assessed under microfluidic conditions and, next, transposed to a lab-scale mesofluidic reactor connected with an in-line NMR spectrometer for real-time reaction monitoring. Substituted γ-butyrolactones, including spiro derivatives with unique structural features, were obtained with quantitative conversion of the starting materials and in 47–76% isolated yields. The model photoaddition of isopropanol to fumaric acid was next successfully transposed in a pilot-scale continuous-flow photoreactor to further demonstrate scalability.
† Center for Integrated Technology and Organic Synthesis, Department of Chemistry, University of Liège, B-4000 Liège (Sart Tilman), Belgium

Romaric Gérardy
PhD Student at Université de Liège
Center for Integrated Technology and Organic Synthesis (CiTOS)
Université de Liège, Liège Area, Belgium

XStruct, Department of Chemistry, Ghent University, Krijgslaan 281-S3, B-9000 Ghent, Belgium
Kristof.VanHecke@UGent.be
Group leader of the XStruct group

Corning Reactor Technologies, Corning SAS, 7 bis Avenue de Valvins, CS 70156 Samois sur Seine, 77215 Avon Cedex, France

Marc Winter
Senior Application Engineer - Advanced-Flow(tm) Reactors
CorningFontainebleau, France
Corning Reactor Technologies, Corning SAS, 7 bis Avenue de Valvins, CS 70156 Samois sur Seine, 77215 Avon Cedex, France

Clemens Horn, Senior Research Scientist
////////////////////



No comments:
Post a Comment