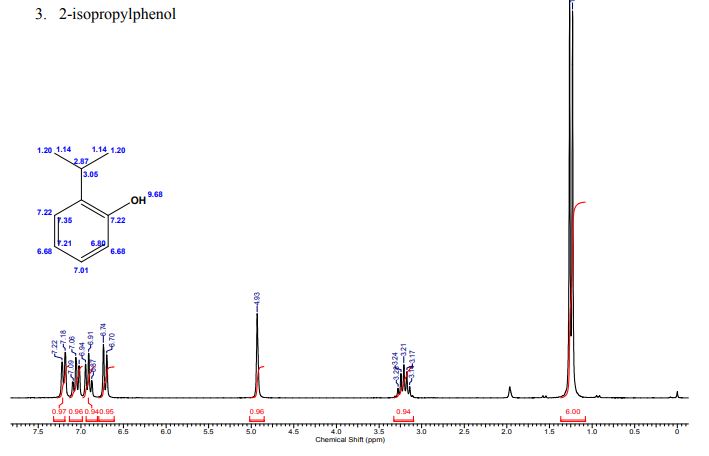
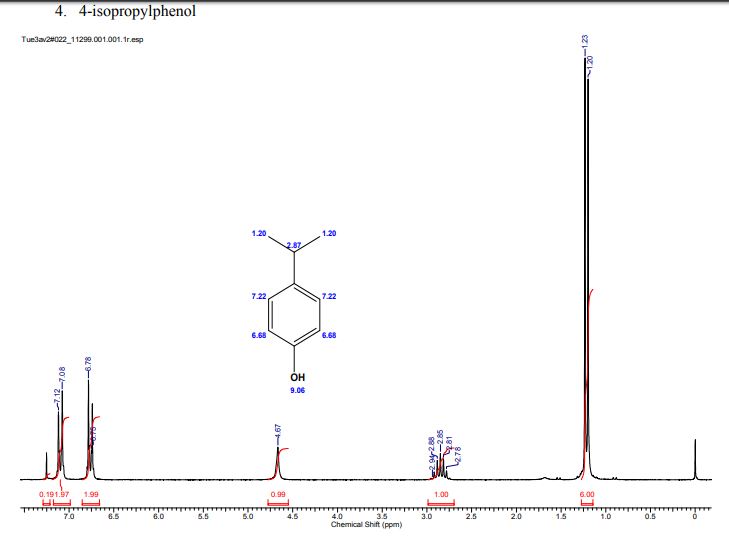
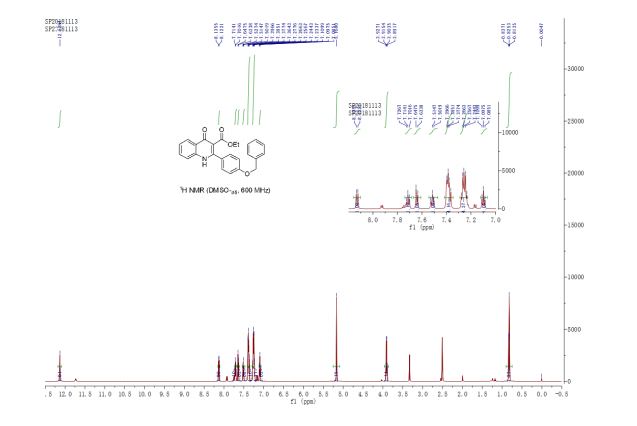
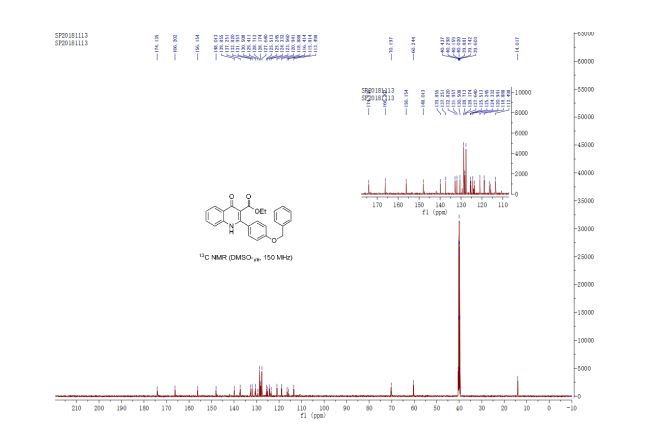
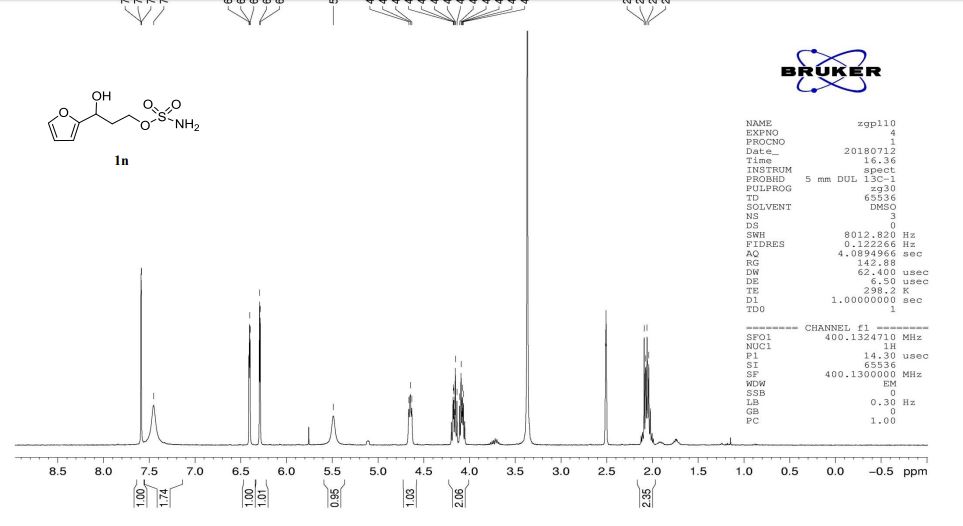
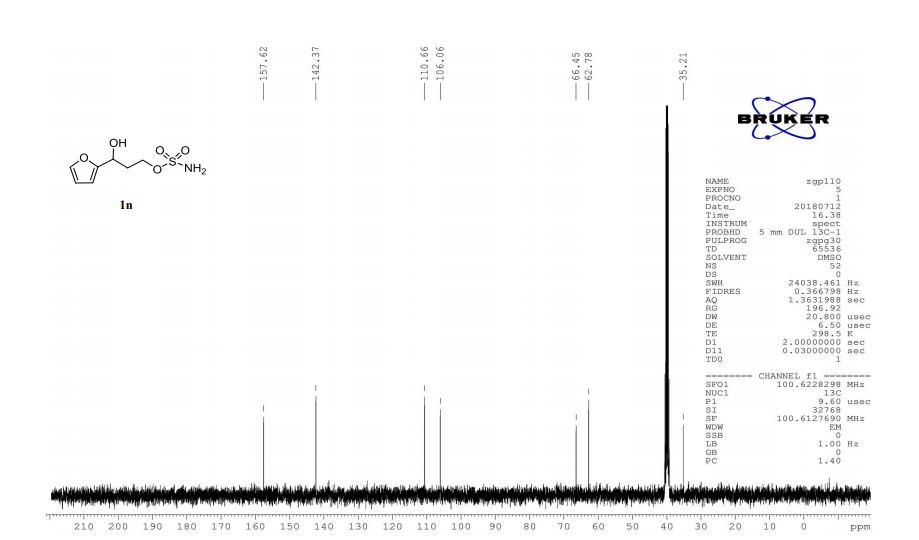
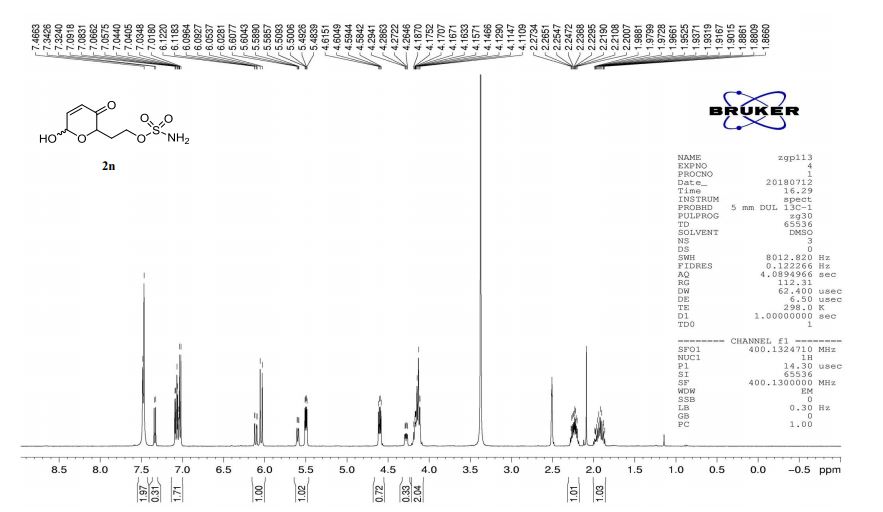
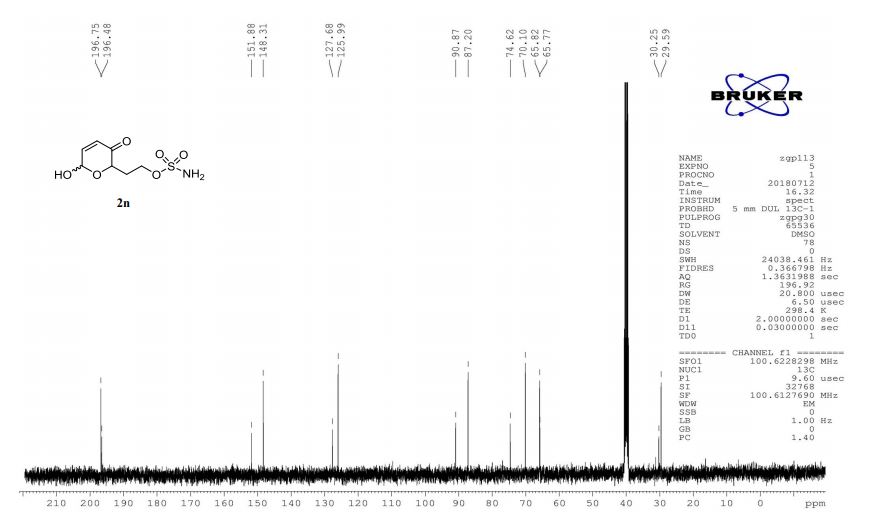
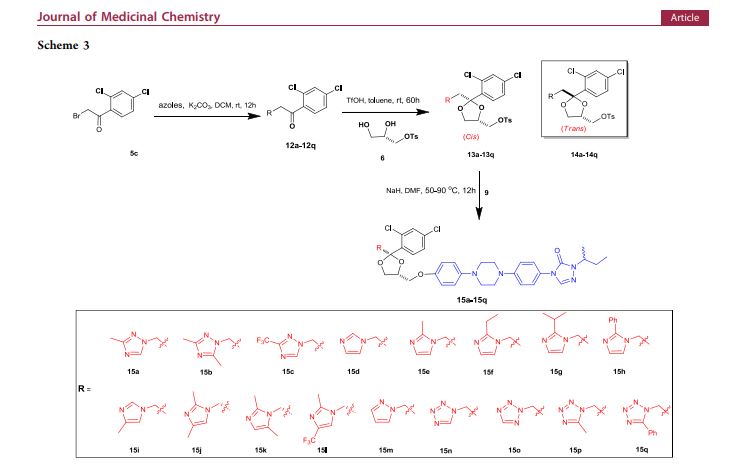
A multistep sequential flow synthesis of isopropyl phenol is demonstrated, involving 4-step exothermic, endothermic, and temperature sensitive reactions such as nitration, reduction, diazotization, and high temperature hydrolysis. Nitration of cumene with fuming nitric acid produces 2- and 4-nitrocumene which are converted into respective cumidines by the hydrogenation using Pd/Ni catalyst in H-cube with gravity separation. Hydrolysis of in situ generated diazonium salts in the boiling acidic conditions is carried out using integration of flow and microwave-assisted synthesis. 58% of 4-isopropyl phenol was obtained. The sequential flow synthesis can be applied to synthesize other organic compounds involving this specific sequence of reactions.
Telescoped Sequence of Exothermic and Endothermic Reactions in Multistep Flow Synthesis
Chemical Engineering & Process Development Division, CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.8b00008
*Phone: +91-20-25902153, E-mail: aa.kulkarni@ncl.res.in.

Dr.Amol A. Kulkarni
Chemical Engineering & Process Development
CSIR-National Chemical Laboratory
Chemical Engineering & Process Development
CSIR-National Chemical Laboratory

Dr. Amol A. Kulkarni is a Scientist in the Chemical Engineering Division at the National Chemical Laboratory. He did his B. Chem. Eng. (1998), M. Chem. Eng (2000) and Ph.D. in chemical engineering (2003) all from the University Dept. of Chem. Technology (UDCT, Mumbai). In 2004 he worked at the Max Planck Institute-Magdeburg (Germany) as a Alexander von Humboldt Research Fellow. At NCL he is driving a research program on the design of microreactors and exploring their applications for continuous syntheses including of nanoparticles. He has been awarded with the Max-Planck-Visiting Fellowship from the Max-Planck-Society, Munich for 2008-2011. His research areas include: (i) design and applications of microreactors, (ii) design of multiphase reactors, (iii) experimental and computational fluid dynamics, and (iv) nonlinear dynamics of coupled systems. He is an active member of Initiative for Research and Innovation in Science (IRIS) supported by Intel’s Education Initiative to organize National Science Fair and popularize science in India.

Yachita Sharma
Location Pune, India
Yachita Sharma is a PhD student at CSIR-National Chemical Laboratory, Pune (India). She received her MSc in Applied Organic Chemistry in 2010. Her work focuses on exploring the continuous flow synthesis involving exothermic reactions and their integration.

Arun Nikam
Location, Pune, India
Email: arun11nikam@gmail.com
Arun was born and raised in Koregaon, Maharashtra, India. He completed his bachelors and masters in chemical sciences from Shivaji Unversity, Kolhapur, India. Currently, He is pursuing his Ph. D. under the supervision of Dr. Amol A. Kulkarni and Dr. B. L. V. Prasad. His work mainly focuses on flow synthesis of nanoparticles, drug formulation, and polymers. He develops new synthesis procedures and translates into flow chemistry to increase productivity with excellent control over the quality of the product. He is also exploring the application of nanoparticles in catalysis, electronics and pharmaceutical fields. He specializes in microwave-assisted continuous flow synthesis of nanomaterial and organic intermediate. Apart from his research, he actively pursues Yoga and spirituality. He also likes to play volleyball and has competed in inter CSIR tournaments.
Arun was born and raised in Koregaon, Maharashtra, India. He completed his bachelors and masters in chemical sciences from Shivaji Unversity, Kolhapur, India. Currently, He is pursuing his Ph. D. under the supervision of Dr. Amol A. Kulkarni and Dr. B. L. V. Prasad. His work mainly focuses on flow synthesis of nanoparticles, drug formulation, and polymers. He develops new synthesis procedures and translates into flow chemistry to increase productivity with excellent control over the quality of the product. He is also exploring the application of nanoparticles in catalysis, electronics and pharmaceutical fields. He specializes in microwave-assisted continuous flow synthesis of nanomaterial and organic intermediate. Apart from his research, he actively pursues Yoga and spirituality. He also likes to play volleyball and has competed in inter CSIR tournaments.
/////////isopropyl phenol, flow chem,
“ALL FOR DRUGS” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent
READ
ANTHONY MELVIN CRASTO
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
CALL +919323115463 INDIA
//////////////