Catalyst- and Additive-Free Baeyer−Villiger-type Oxidation of α-Iodocyclopentenones to α-Pyrones: Using Air as the Oxidant
Abstract
An efficient synthetic approach for the synthesis of α-pyrones via Baeyer−Villiger-type oxidation of α-iodocyclopentenones through a catalyst- and additive-free system using air as an environmentally benign oxidant is described. The reaction exhibits excellent functional group compatibility and provides a simple and efficient protocol for the construction of highly functionalized α-pyrones under mild reaction conditions.
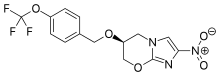
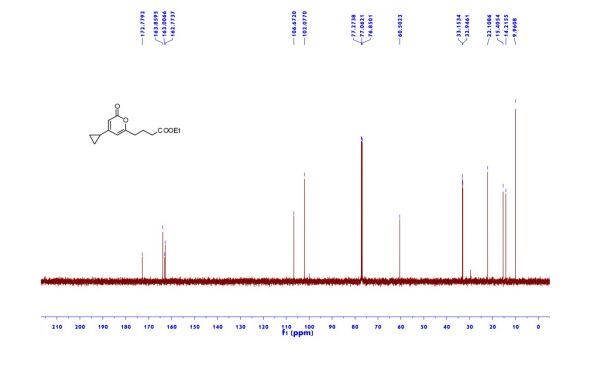
Ethyl 4-(4-cyclopropyl-2-oxo-2H-pyran-6-yl)butanoate (2aa) Product 2aa was obtained as yellow oil in 50% yield (38 mg) following the general procedure; 1H NMR (600 MHz, CDCl3) δ 5.84 (s, 1H), 5.61 (s, 1H), 4.13-4.09 (m, 2H), 2.48 (t, J = 7.3 Hz, 2H), 2.33 (td, J = 7.3, 2.3 Hz, 2H), 1.97-1.94 (m, 2H), 1.66-1.63 (m, 1H), 1.26-1.22 (m, 3H), 1.07-1.05 (m, 2H), 0.80-0.79 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 172.8, 163.9, 163.0, 162.8, 106.7, 102.1, 60.5, 33.2, 33.0, 22.1, 15.4, 14.2, 10.0; HRMS (ESI) calcd. for C14H18O4Na [M+Na]+ : 273.1097, found: 273.1101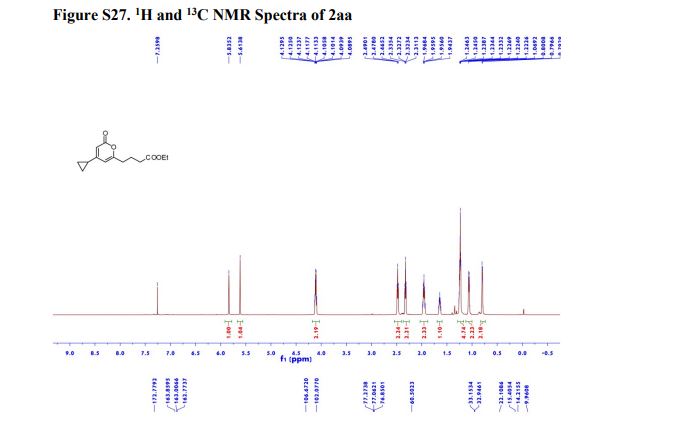
////////////
https://pubs.rsc.org/en/Content/ArticleLanding/2019/GC/C9GC02725D?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract