NOESY experiment of diastereomer 18β (3S, 5S)
(3S,5S)-10-(4-Nitrophenyl)-4,5a,6,7,8,10-hexahydro-5H-pyrrolo-[1,2-a]thieno[3,2-e][1,4]diazepin-2-one (18β)
(3S,5S)-10-(4-Nitrophenyl)-4,5a,6,7,8,10-hexahydro-5H-pyrrolo-[1,2-a]thieno[3,2-e][1,4]diazepin-2-one (18β): Pale orange wax, 62%(104.0 mg).
[α]D29 = +8.8 (c = 1.0, MeOH).
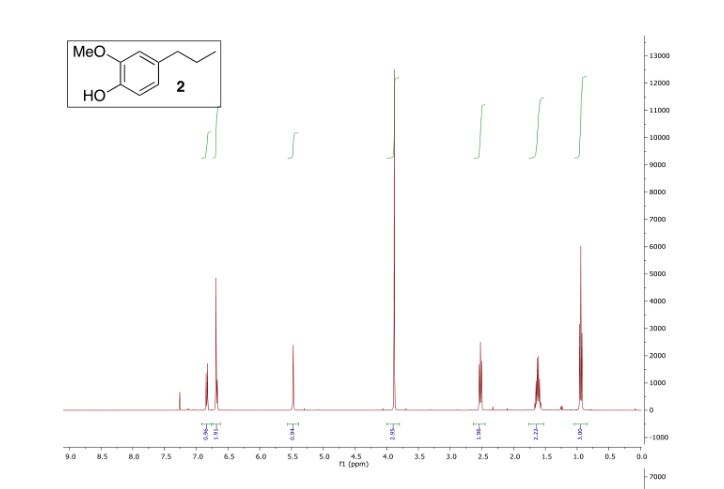
1H NMR (CDCl3,300 MHz): δ = 1.83–1.92 (m, 2 H), 2.14–2.21 (m, 2 H), 2.90 (td, J= 8.7 Hz, 1 H), 3.41–3.47 (m, 1 H), 3.91 (t, J = 6.6 Hz, 1 H), 5.66(s, 1 H), 7.17–7.24 (m, 3 H), 7.44 (d, J = 8.7 Hz, 2 H), 8.18 (t, J =8.7 Hz, 2 H) ppm.
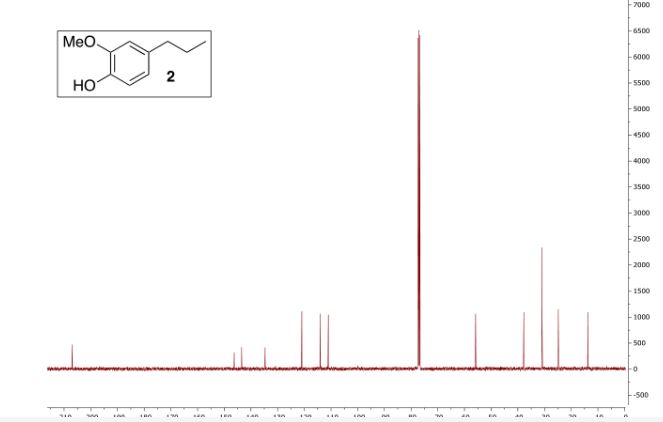
13C NMR (CDCl3, 75 MHz): δ = 24.88, 27.44,56.40, 63.70, 82.66, 111.10, 120.28, 124.26 (2 C), 125.37, 127.05 (2C), 135.23, 146.30, 147.97, 173.56 ppm.
LC–MS (ESI+): m/z =330.1 [M + H]+. HRMS: calcd. for C16H16N3O3S 330.0912 [M +
H]+; found 330.0914.
DOI: 10.1002/ejoc.201500943
Eur. J. Org. Chem. 2015, 7146–7153
////////////