Mephedrone hydrochloride
4-Methylmethcathino
2-(methylamino)-1-(
2-(methylamino)-1-(
2-(METHYLAMINO)-1-(
- CAS Number 1189726-22-4
- Empirical Formula C11H15NO·HCl
- Molecular Weight 213.70
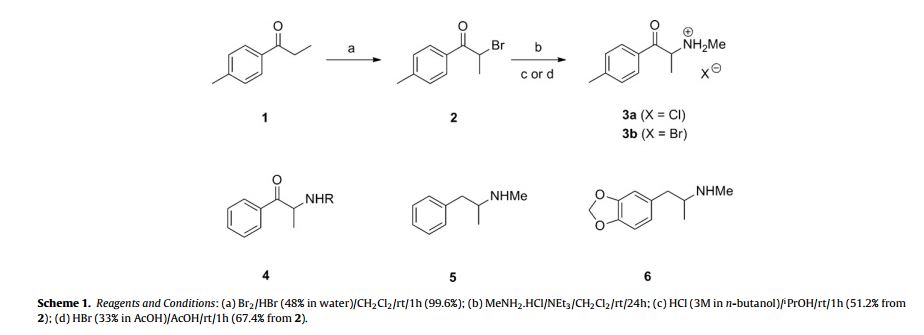
Synthesis of (±)-4 -methyl-2-bromopropiophenone (2) [1,2]
(±)-4 -methyl-2-bromopropiophenone (22.6 g, 99.6%) as colourless prisms. Mpt. (Et2O) 76–77 ◦C (lit. [2] 75–77 ◦C);
Rf [SiO2, EtOAc:n-hexane (1:3)] = 0.79;
1H NMR (400 MHz, 25 ◦C, CDCl3) ı = 7.91 (2H, d, J = 8.3 Hz, AA BB ), 7.27 (2H, d, J = 8.3 Hz, AA BB ), 5.28 (1H, q, J = 7.0 Hz, CH(Br)CH3), 2.42 (3H, s, ArCH3) and 1.86 ppm (3H, d, J = 7.0 Hz, CH(Br)CH3);
13C NMR (400 MHz, 23 ◦C, CDCl3) ı = 193.1 (C O), 144.8 (ArC), 131.6 (ArC), 129.5 (2×ArCH), 129.1 (2× ArCH), 41.6 (CH(Br)CH3), 21.8 (ArCH3) and 20.3 ppm (CH(Br)CH3); GCMS (EI, 70 eV): tR = 5.09 min; m/z = 225.5 (5, [MBr79]+), 227.5 (5, [MBr81]+), 118.3 (100), 108.4 (12), 90.5 (85) and 64.5 (70%). The br
1 D.M. Kalendra, B.R. Sickles, Diminished reactivity of ortho-substituted phenacyl bromides toward nucleophilic displacement, J. Org. Chem. 68 (2003) 1594–1596.
[2] K. Ogawa, T. Terada, T. Yamazaki, S. Yamada, T. Honna, S. Ohta, M. Okamoto, Synthesis of novel 5- and 6-substituted 3-arylidene-1 4-oxathiin-2(3H)-ones, J. Chem. Soc. (Perkin Trans. 1) 11 (1985) 2417–2423.
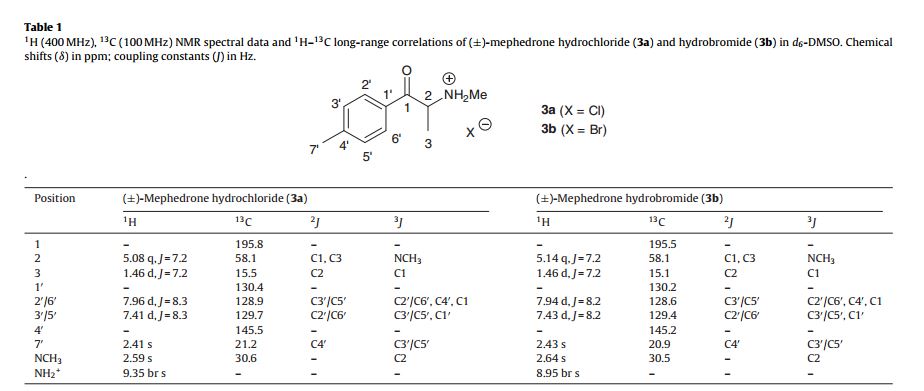
Synthesis of (±)-4 -methylmethcathinone hydrochloride [(±)-mephedrone hydrochloride] (3a) [3,4]
(±)-4 -methylmethcathinone hydrochloride (1.09 g, 51.2% from 2) as a colourless powder. Mpt. (acetone) 251.18 ◦C;
Rf [SiO2, EtOAc:n-hexane (1:3)] = 0.11; []D22 = 0 (c = 0.5 g/100 mL in MeOH);
found: C, 61.61; H, 7.35; N, 6.17. C11H16ClNO requires C, 61.82; H, 7.55 and N, 6.55%;
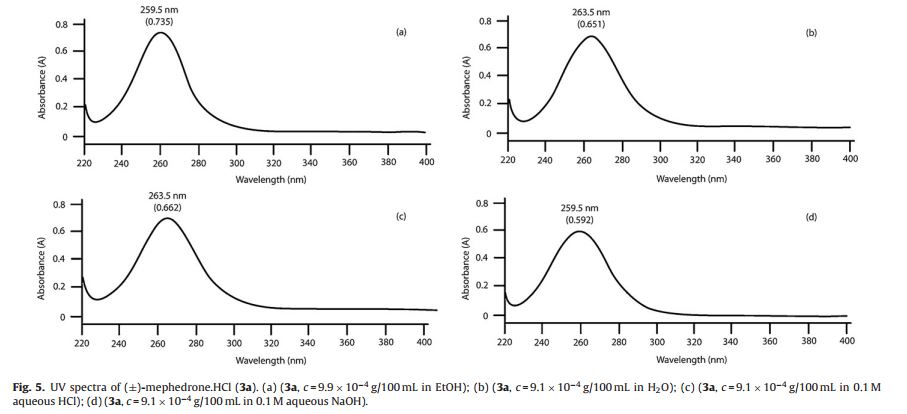
UV (EtOH): max = 259.5 nm (A = 0.735, c = 9.95 × 10−4 g/100 mL);
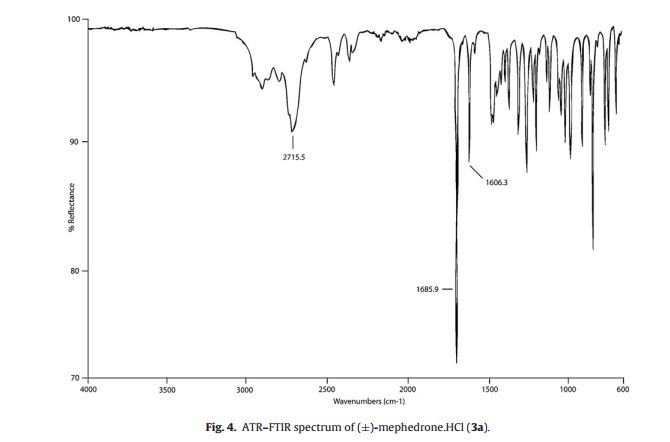
IR (ATR–FTIR): 2717.5 (NH2 +), 1689.5 (C O), 1606.3 cm−1 (C C);
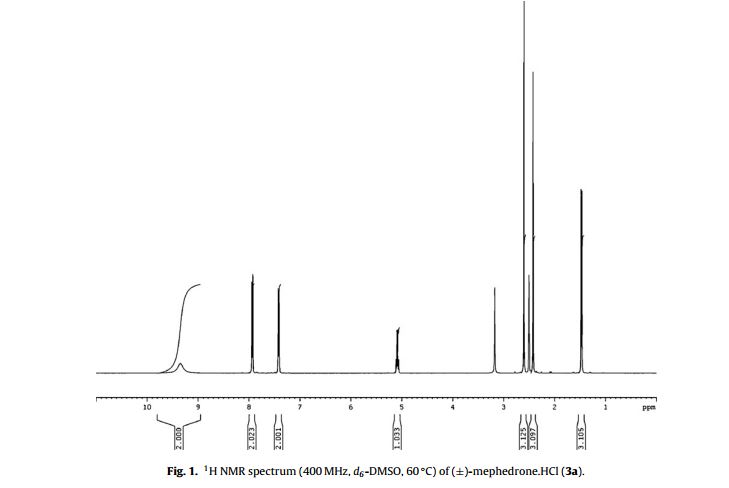
1H NMR (400 MHz, 60 ◦C, d6- DMSO) ı = 9.35 (2H, br s, CH(NH2 +CH3)CH3); 7.96 (2H, d, J = 8.3 Hz, AA BB ), 7.41 (2H, d, J = 8.3 Hz, AA BB ), 5.08 (1H, q, J = 7.2 Hz, CH(NH2 +CH3)CH3), 2.59 (3H, s, CH(NH2 +CH3)CH3), 2.41 (3H, s, ArCH3) and 1.46 ppm (3H, d, J = 7.2 Hz, CH(NH2 +CH3)CH3);
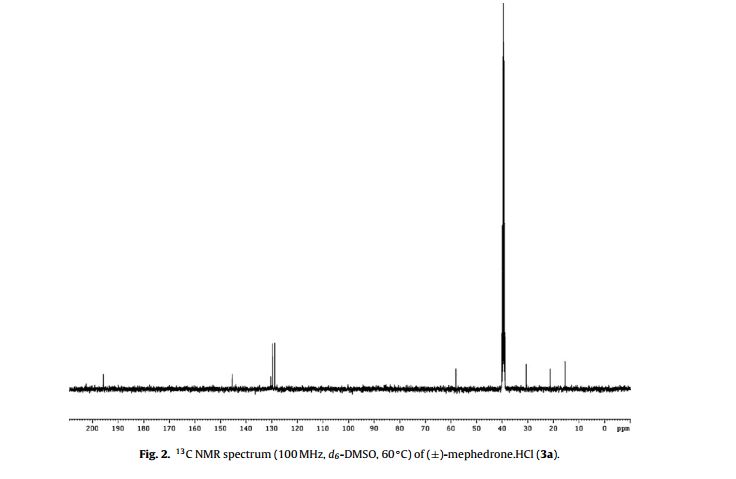
13C NMR (400 MHz, 60 ◦C, d6-DMSO) ı = 195.8 (C O, C1), 145.5 (ArC, C4 ), 130.4 (ArC, C1 ), 129.7 (2× ArCH, C3 /C5 ), 128.9 (2× ArCH, C2 /C6 ), 58.1 (CHCH3, C2), 30.6 (NCH3,), 21.2 (ArCH3, C7 ) and 15.5 ppm (CHCH3, C3);
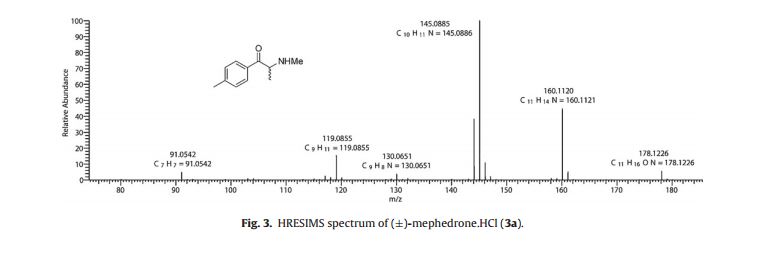
LRMS (ESI+, 70 eV): m/z = 178 (6%, [M+H]+), 160 (47), 145 (100), 130 (7), 119 (16) and 91 (5);
HRMS (ESI+, 70 eV) calculated for [M+H] C11H16NO: 178.1226, found: 178.1226. 2.3. Synthes
3 A. Camilleri, M.R. Johnston, M. Brennan, S. Davis, D.G.E. Caldicott, Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alphaphthalimidopropiophenone and N-ethylcathinone, Forensic Sci. Int. 197 (2010) 59–66.
[4] J. Deruiter, L. Hayes, A. Valaer, C.R. Clark, F.T. Noggle, Methcathinone and designer analogues: synthesis, stereochemical analysis, and analytical properties, J. Chromatogr. Sci. 32 (1994) 552–564.
E.Y. Santali et al. / Journal of Pharmaceutical and Biomedical Analysis 56 (2011) 246–255
///////////(±)-4 -methylmethcathinone hydrochloride, (±)-mephedrone hydrochloride, 4-methylmethcathinone hydrochloride, mephedrone hydrochloride,