cas 99395-88-7
C9H9NO2, 163.2
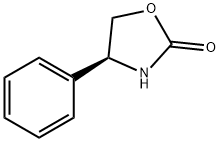
- 2-Oxazolidinone, 4-phenyl-, (S)-
- (+)-4-Phenyl-2-oxazolidinone
- (4S)-4-Phenyl-1,3-oxazolidin-2-one
- (4S)-4-Phenyl-2-oxazolidinone
- (4S)-4-Phenyloxazolidin-2-one
- (S)-(+)-4-Phenyl-2-oxazolidinone
- (S)-4-Phenyl-1,3-oxazolidin-2-one
- (S)-4-Phenyl-2-oxazolidinone
- (S)-POZ
- (S)-POZ
mp 124-126 °C
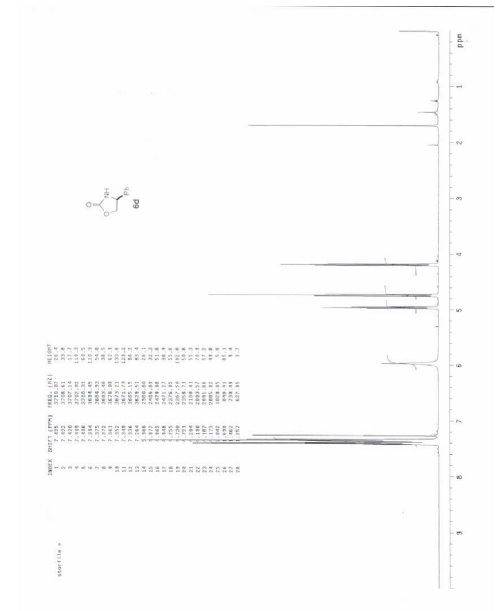
| (S)-(+)-4-Phenyl-2-oxazolidinone Property | |||||||||
|
MacNevin, Christopher J.; Journal of Organic Chemistry 2008, V73(4), P1264-1269
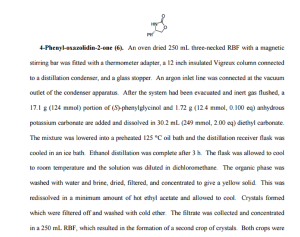
4-Phenyl-oxazolidin-2-one (6). An oven dried 250 mL three-necked RBF with a magnetic stirring bar was fitted with a thermometer adapter, a 12 inch insulated Vigreux column connected to a distillation condenser, and a glass stopper. An argon inlet line was connected at the vacuum outlet of the condenser apparatus. After the system had been evacuated and inert gas flushed, a 17.1 g (124 mmol) portion of (S)-phenylglycinol and 1.72 g (12.4 mmol, 0.100 eq) anhydrous potassium carbonate are added and dissolved in 30.2 mL (249 mmol, 2.00 eq) diethyl carbonate. The mixture was lowered into a preheated 125 °C oil bath and the distillation receiver flask was cooled in an ice bath. Ethanol distillation was complete after 3 h. The flask was allowed to cool to room temperature and the solution was diluted in dichloromethane. The organic phase was washed with water and brine, dried, filtered, and concentrated to give a yellow solid. This was redissolved in a minimum amount of hot ethyl acetate and allowed to cool. Crystals formed which were filtered off and washed with cold ether. The filtrate was collected and concentrated in a 250 mL RBF, which resulted in the formation of a second crop of crystals. Both crops were combined to give 17.0 g (84%) off-white crystalline solid.
Rf = 0.42 (95:5 DCM/MeOH); mp 124-126 °C; [J] 23D +48.1 (c 1.00, CHCl3) {ref.1 [J] 23D +49.5 (c 2.10, CHCl3)};
1 H NMR (400 MHz, CDCl3) > 7.43-7.33 (m, 5H), 6.27 (bs, 1H), 4.96 (t, 1H, J = 8.0 Hz), 4.73 (t, 1H, J = 8.8 Hz), 4.18 (dd, 1H, J = 8.8, 7.2 Hz);
13C NMR (100 MHz, CDCl3) > 160.2, 139.7, 129.3, 128.9, 126.2, 72.7, 56.5;
IR (film): 3246, 1736, 1704, 1399, 1234, 1097, 923, 695 cm-1;
HRMS-ESI m/z 164.0703 ([M+H]+ , C9H10NO2 requires 164.0706).
ref 1 Evans, D. A.; Sjorgen, E. B. Tetrahedron Lett. 1985, 26, 3783.
next.................
Dinsmore, Christopher J.; Organic Letters 2004, V6(17), P2885-2888
Dinsmore, Christopher J.; Organic Letters 2004, V6(17), P2885-2888
(4S)-4-Phenyl-1,3-oxazolidin-2-one (6d):
3 Analytical LCMS: single peak (1.07 min, CH3CN/H2O/0.05% TFA, 4 min gradient) 97.3% pure by UV (215 nm);
1H NMR (500 MHz, CDCl3) d 7.33-7.42 (m, 5H), 5.97 (br s, 1H), 4.96 (br t, J = 8 Hz, 1H), 4.74 (t, J = 8.6 Hz, 1H), 4.19 (dd, J = 8.6 and 6.9 Hz, 1H);
m/z (ES+ ) = 164.3 (MH+ );
HRMS (APCI) exact mass calcd for C9H10NO2 (M+H+ ): 164.0706; found 164.0712.