TAVABOROLE
- AN 2690
- AN-2690
- AN2690
- UNII-K124A4EUQ3
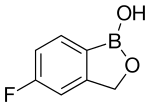
5-Fluoro-2,1-benzoxaborol-1(3H)-ol;
1,3-Dihydro-5-fluoro-1-hydroxy-2,1-benzoxaborole
MOLECULAR FORMULA C7H6BFO2
MOLECULAR WEIGHT 151.9
SPONSOR Anacor Pharmaceuticals, Inc.
CAS REGISTRY NUMBER 174671-46-6
Mp 118-120° C…..US20070265226
1H NMR (300 MHz, DMSO-d6) δ (ppm) 4.95 (s, 2H), 7.15 (m, 1H), 7.24 (dd, J=9.7, 1.8 Hz, 1H), 7.74 (dd, J=8.2, 6.2 Hz, 1H), 9.22 (s, 1H)
FDA APPROVED JULY 2 2014………..“FDA Approves Anacor Pharmaceuticals’ KERYDIN™ (Tavaborole) Topical Solution, 5% for the Treatment of Onychomycosis of the Toenails”. Market Watch. July 8, 2014.
Has antifungal activity.
U.S. Patent Nos. 7,767,657 and 7,582,621
The US Food and Drug Administration (FDA) 2014 JULY 8 ratified the Anacor’s Kerydin (5% Tavaborole solution) for the topical treatment of nail fungal infections. Tavaboroleindications of toenail fungus Trichophyton rubrum or Trichophyton rubrum infections.Instructions recommended once a day for toenail infections, treatment for 48 weeks, on the recommendation of Anacor, and do not need to nail debridement.
I tis an oxaborole antifungal used topically, as a 5% w/w solution, for the treatment of onychomycosis of the toenails due to Trichophyton rubrumor T. mentagrophytes. It is applied to the affected toenail once daily for 48 weeks.
Ingrowing toenails and application site reactions including exfoliation, erythema, and dermatitis have been reported during use.
COSY NMR PREDICT

Tavaborole (AN2690, trade name Kerydin) is a topical antifungal medication for the treatment of onychomycosis, a fungal infectionof the nail and nail bed. Tavaborole began its Phase 3 trials in December 2010[1] and was approved in July 2014.[2] Tavaborole inhibits an essential fungal enzyme, Leucyl-tRNA synthetase, or LeuRS, required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the fungal infection. No treatment-related systemic side effects were observed in any of its clinical trials.
Tavaborole is the first oxygen boron used to treat toenail infections dioxolane (oxaborole) antifungal agents, located in Palo Alto, Anacor focuses on boron-based drug development and production, according to the latest news, Tavaborole future also be used to infect fingernails. Wedbush Securities analyst predicts that next year the drug sales in the United States for $ 16 million, by 2021 will reach peak sales of $ 347 million.
Gram-negative bacteria cause approximately 70% of the infections in intensive care units. A growing number of bacterial isolates responsible for these infections are resistant to currently available antibiotics and to many in development. Most agents under development are modifications of existing drug classes, which only partially overcome existing resistance mechanisms. Therefore, new classes of Gram-negative antibacterials with truly novel modes of action are needed to circumvent these existing resistance mechanisms. We have previously identified a new a way to inhibit an aminoacyl-tRNA synthetase, leucyl-tRNA synthetase (LeuRS), in fungi via the oxaborole tRNA trapping (OBORT) mechanism.
Herein, we show how we have modified the OBORT mechanism using a structure-guided approach to develop a new boron-based antibiotic class, the benzoxaboroles, which inhibit bacterial leucyl-tRNA synthetase and have activity against Gram-negative bacteria by largely evading the main efflux mechanisms in Escherichia coli and Pseudomonas aeruginosa. The lead analogue, is active against Gram-negative bacteria, including Enterobacteriaceaebearing NDM-1 and KPC carbapenemases, as well as P. aeruginosa. This novel boron-based antibacterial, has good mouse pharmacokinetics and was efficacious against E. coli and P. aeruginosa in murine thigh infection models, which suggest that this novel class of antibacterials has the potential to address this unmet medical need.
Anacor continued development on that drug, tavaborole, and filed for FDA approval in July. The FDA will review the phase 3 trial data and issue a decision on July 29, 2014.
If approved, Anacor hopes tavaborole’s ability to clear onychomycosis in 10% of treated patients will be enough to win market share away from generic Lamisil and generic topical Pentac. While Lamisil cleared the fungus in 38% of patients, it’s been associated with rare cases of liver failure. And Pentac requires frequent debridement of the nail and only clears the fungus in 5.5% to 8.5% of patients.
Tavaborole is a novel, topical antifungal medication being developed for the topical treatment of onychomycosis, a nail fungus infection, which affects seven to ten percent of the U.S. population. Early studies show AN-2690 penetrates the nail effectively and has robust activity against dermatophytes, which cause onychomycosis.
1H NMR FROM NET
CLICK ON IMAGE FOR CLEAR VIEW
1H NMR PREDICT


……………………………………………………………………………
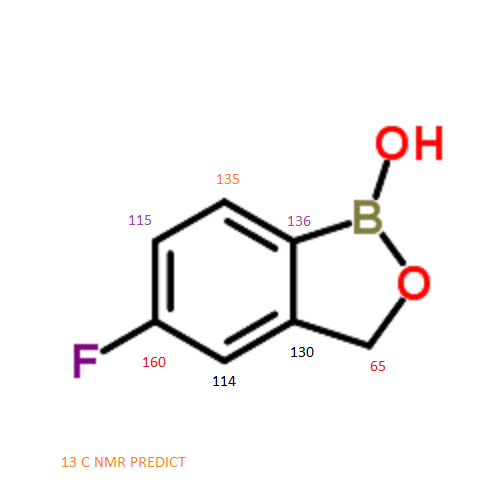
13 C NMR PREDICT

ARTICLE
July 18, 2013
Abstract Accepted for Oral Presentation at the 2013 American Podiatric Medical Association Annual Scientific Meeting
PALO ALTO, Calif.–(BUSINESS WIRE)– Anacor Pharmaceuticals (NASDAQ:ANAC) announced today that its abstract “Pivotal Phase 3 Safety and Efficacy Results of Tavaborole (Formerly AN2690), a Novel Boron-Based Molecule for the Topical Treatment of Toenail Onychomycosis” was accepted for oral presentation at the 2013 APMA Annual Scientific Meeting (The National) to be held in Las Vegas, Nevada. Max Weisfeld, DPM, will present the data from tavaborole’s Phase 3 studies on Monday, July 22, 2013 during the Evidence-Based Medicine and Oral Abstracts session.
As announced earlier this year, tavaborole achieved statistically significant and clinically meaningful results on all primary and secondary endpoints in two Phase 3 pivotal studies without concomitant debridement. Anacor is seeking approval for tavaborole from the Food and Drug Administration (FDA) and will file a New Drug Application imminently. Currently, there is only one FDA-approved topical treatment for onychomycosis, a fungal infection of the nail and nail bed, which affects approximately 35 million people in the United States.
“I’m impressed with tavaborole’s safety and efficacy data. There is no FDA-approved topical treatment for onychomycosis with tavaborole’s range of efficacy and ability to penetrate the nail to reach the site of the infection,” said Dr. Weisfeld. “Tavaborole’s Phase 3 results demonstrate its ability to clear the nail and eliminate the infection which is important to both patients and the physicians who treat them. In addition, tavaborole is easy to apply and dries quickly which makes it convenient for patients to use.”
“We are pleased to present these positive data at the APMA’s Annual Scientific Meeting, the leading annual meeting of podiatrists. As we seek FDAapproval for tavaborole, we look forward to developing relationships with podiatrists to potentially offer them a new treatment option for the large number of patients who seek treatment for onychomycosis,” said David Perry, Chief Executive Officer of Anacor Pharmaceuticals.
About the Studies
Anacor conducted two separate Phase 3 studies of tavaborole on patients with distal subungual onychomycosis affecting 20 to 60 percent of the target great toenail. Approximately 600 patients aged 18 years and older with no upper age limit (the oldest subject was 88 years old) were enrolled in each study and randomized two-to-one to receive either tavaborole or the vehicle control. Patients were instructed to apply tavaborole solution or the vehicle to the toenail once daily for 48 weeks.
A copy of the presentation will be available on Anacor’s website following the oral session.
About Anacor Pharmaceuticals
Anacor is a biopharmaceutical company focused on discovering, developing and commercializing novel small-molecule therapeutics derived from its boron chemistry platform. Anacor has discovered eight compounds that are currently in development. Its two lead product candidates are topically administered dermatologic compounds — tavaborole, a topical antifungal for the treatment of onychomycosis, and AN2728, a topical anti-inflammatory PDE-4 inhibitor for the treatment of atopic dermatitis and psoriasis. In addition to its two lead programs, Anacor has discovered three other wholly-owned clinical product candidates — AN2718 and AN2898, which are backup compounds to tavaborole and AN2728, respectively, and AN3365 an antibiotic for the treatment of infections caused by Gram-negative bacteria. We have discovered three other compounds that we have out-licensed for further development — two compounds for the treatment of animal health indications that are licensed to Eli Lilly and Company and AN5568, also referred to as SCYX-7158, for human African trypanosomiasis (HAT, or sleeping sickness), which is licensed to Drugs for Neglected Diseases initiative, or DNDi. We also have a pipeline of other internally discovered topical and systemic boron-based compounds in development. For more information, visit http://www.anacor.com.
…………………………………………………………………………………….
Patents
WO 1995033754
WO 2004009578….
WO 2006089067
WO 2008025543
…………………………..
SYNTHESIS
Drugs Fut 2006, 31(8): 667
J Med Chem 2006, 49(15): 4447
……………………………
Reference:
ELI LILLY AND COMPANY Patent: WO2004/9578 A2, 2004 ; Location in patent: Page 36-37 ; WO 2004/009578 A2………………………
PATENT
Anacor Pharmaceuticals Patent: US2007/265226 A1, 2007 ; Location in patent: Page/Page column 59 ;
http://www.google.com/patents/US20070265226
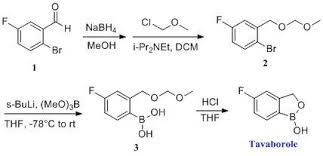
1,3-Dihydro-5-fluoro-1-hydroxy-2,1-benzoxaborole (19b)
To a solution of 5b (73.2 g, 293 mmol) in dry THF (400
mL) was added n-butyllithium (1.6 M in hexanes; 200 mL) over 45 min at
−78° C. under nitrogen atmosphere. Anion precipitated. After 5 min,
(i-PrO)3B (76.0 mL, 330 mmol) was added over 10 min, and the
mixture was allowed to warm to room temperature over 1.5 h. Water and 6 N
HCl (55 mL) were added, and the solvent was removed under reduced
pressure to about a half volume. The mixture was poured into ethyl
acetate and water. The organic layer was washed with brine and dried
over anhydrous Na2SO4. The solvent was removed
under reduced pressure. To a solution of the residue in tetrahydrofuran
(360 mL) was added 6 N HCl (90 mL), and the mixture was stirred at 30°
C. overnight. The solvent was removed under reduced pressure to about a
half volume. The mixture was poured into ethyl acetate and water. The
organic layer was washed with brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the residue was treated with i-Pr2O/hexane to give 19b (26.9 g, 60%) as a white powder:
mp 118-120° C.; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 4.95 (s, 2H), 7.15 (m, 1H), 7.24 (dd, J=9.7, 1.8 Hz, 1H), 7.74 (dd, J=8.2, 6.2 Hz, 1H), 9.22 (s, 1H);
ESI-MS m/z 151 (M−H)−;
HPLC purity 97.8%; Anal (C7H6BFO2) C, H.
…………………
Gunasekera, Dinara S.; Gerold, Dennis J.; Aalderks, Nathan S.; Chandra, J. Subash; Maanu, Christiana A.; Kiprof, Paul; Zhdankin, Viktor V.; Reddy, M. Venkat Ram Tetrahedron, 2007 , vol. 63, # 38 p. 9401 – 9405
…………………….
Baker, Stephen J.; Zhang, Yong-Kang; Akama, Tsutomu; Lau, Agnes; Zhou, Huchen; Hernandez, Vincent; Mao, Weimin; Alley; Sanders, Virginia; Plattner, Jacob J. Journal of Medicinal Chemistry, 2006 , vol. 49, # 15 p. 4447 – 4450
………………..
Ding, Charles Z.; Zhang, Yong-Kang; Li, Xianfeng; Liu, Yang; Zhang, Suoming; Zhou, Yasheen; Plattner, Jacob J.; Baker, Stephen J.; Liu, Liang; Duan, Maosheng; Jarvest, Richard L.; Ji, Jingjing; Kazmierski, Wieslaw M.; Tallant, Matthew D.; Wright, Lois L.; Smith, Gary K.; Crosby, Renae M.; Wang, Amy A.; Ni, Zhi-Jie; Zou, Wuxin; Wright, Jon Bioorganic and Medicinal Chemistry Letters, 2010 , vol. 20, # 24 p. 7317 – 7322
…………..
PATENT
US20050261277
PREPARATION 13 5-Fluoro-3H-benzo[c][1,2)oxaborol-1-ol
Dissolve 1-bromo-2-(1-ethoxy-ethoxymethyl)-4-fluoro-benzene(5.4 g, 19.5 mmol) in dry THF (100 mL) and cool to −78° C. under nitrogen. Add butyl lithium (2.5M in Hexanes, 10.2 mL, 25.4 mmol) dropwise at −78° C. Upon complete addition, stir the reaction at −78° C. for 10 minutes and then add trimethyl borate (4.4 mL, 39 mmol) and warm the reaction to room temperature. Pour the reaction into 1N HCl (100 mL) and stir for 1 hour. Extract the biphasic mixture with ether three times. Dry the combined organic layers with sodium sulfate, filter and concentrate in vacuo. Triturate the oily residue with cold hexanes to yield 2.1 g (70%) of the title compoud as a white solid.
1H NMR (d6-DMSO)
9.18 (s, 1H),
7.70 (dd, J=8.2, 5.8 Hz, 1H),
7.20 (dd, J=9.5, 2.7 Hz, 1H),
7.11 (m, 1H), 4.92 (s, 1H).
…………………
SEE
http://jpet.aspetjournals.org/content/early/2012/11/28/jpet.112.200030.full.pdf
………………………………..
SEE
Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis.
Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MR, Sanders V, Plattner JJ.
J Med Chem. 2006 Jul 27;49(15):4447-50.
Boron-containing inhibitors of synthetases.
J Med Chem. 2006 Jul 27;49(15):4447-50.
Boron-containing inhibitors of synthetases.
Baker SJ, Tomsho JW, Benkovic SJ.
Chem Soc Rev. 2011 Aug;40(8):4279-85. doi: 10.1039/c0cs00131g. Epub 2011 Feb 7. Review.
Chem Soc Rev. 2011 Aug;40(8):4279-85. doi: 10.1039/c0cs00131g. Epub 2011 Feb 7. Review.
- Benzoxaborole
antimalarial agents. Part 2: Discovery of fluoro-substituted
7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles.
Zhang YK, Plattner JJ, Freund YR, Easom EE, Zhou Y, Ye L, Zhou H, Waterson D, Gamo FJ, Sanz LM, Ge M, Li Z, Li L, Wang H, Cui H.
Bioorg Med Chem Lett. 2012 Feb 1;22(3):1299-307. doi: 10.1016/j.bmcl.2011.12.096. Epub 2011 Dec 28.
Tavaborole Market Opportunity
Anacor is developing tavaborole specifically to address the current limitations of existing treatment options for onychomycosis. This includes designed leaps forward in both the potential safety and efficacy profile aimed to make the drug a best-in-class therapy. Additionally, management has used the company’s expertise in medicinal chemistry to improve delivery of the compound through the nail plate to the nail bed, the site of onychomycosis infection. For example, preclinical studies indicate that tavaborole is able to penetrate the nail plate 250 times more effectively than ciclopirox.
Tavaborole novel mechanism of action inhibits an essential fungal enzyme, leucyl transfer RNA synthetase, or LeuRS required for protein synthesis. The inhibition of protein synthesis leads to termination of cell growth and cell death, eliminating the fungal infection.
Likewise, the topical dosing was designed to eliminate systemic absorption. Previous preclinical and clinical data shows topical treatment with tavaborole resulted in little or no detectable levels of drug in the blood or urine. No treatment related systemic side effects have been observed in any clinical trials to date. Safety data from the company’s studies to date was recently presented at the 100th National APMA meeting in Washington, DC.
Anacor’s topical solution currently in two phase III trials for onychomycosis. Phase II data with tavaborole suggests efficacy superior to ciclopirox with little to no systemic exposure.
Data from an open-label phase 2 program with tavaborole showed 50% patients using a 7.5% solution saw 2 mm clear nail growth and negative fungal cultures after six months. Roughly 25% of the patients saw 5 mm clear nail growth and negative fungal cultures after six months.I see onychomycosis as a significant market opportunity for Anacor. An estimated 35 million Americans have nail fungus, with about 95% of the infections in the toenail. With efficacy similar to Lamisil, we think Anacor can capture 20% of the market. With a price per course of treatment at around $1,200, I think peak sales of tavaborole are $500 million.
Anacor and partner Merck (NYSE:MRK) met with the U.S. FDA in 2009 to discuss the phase II data. Merck has since returned the rights to tavaborole to Anacor. The original deal was with Schering-Plough in 2007. Merck most likely felt as though tavaborole clashed with existing products or did not have peak sales potential large enough to continue the partnership with Anacor. We see tavaborole as a specialty promoted product, into podiatrists and dermatologists. For a company like Anacor, it’s an attractive first product.
Anacor’s first phase III trial completed enrollment in November 2011. The second phase III trial completed enrollment in December 2011. Data from these trials are expected around the middle of January 2013. Data from the second study is expected six weeks later. Given the positive phase II data noted above, we think odds favor a positive outcome. A benchmark for the trial is the efficacy of Lamisil, which is a complete cure rate of around 35% to 40%, and a mycological cure of around 70% after a typical course of treatment.
I note that on Anacor’s third quarter conference call management noted that they are pleased with the conduct of the trial to date. Specifically, the compliance rate appears to better than management had expected. The trial was designed with a 20% drop-out rate. It looks as though the drop-out rate is only around 13%, at a minimum suggestive of good safety and tolerability, but potentially also a sign that the drug is working.
Conclusion
I’ll note two more important pieces of information for investors. Firstly, besides optimism for tavaborole, Anacor has apipeline of anti-infectant drugs. For this article I discussed only tavaborole. A second article can be dedicated entirely to AN2728 for the treatment of psoriasis and atopic dermatitis. Anacor also has an animal health collaboration with Eli Lilly (NYSE:LLY).
The second important thing to note is Anacor’s cash position. The company reported financial results on November 7, 2012. The company held $36.6 million in cash on the balance sheet as of September 30, 2012. However, in October 2012, the company completed an underwritten public offering of 4.0 million shares of common stock at $6.00 per share to raise net proceeds of $22.7 million. I view the current cash position as sufficient to report data from both phase 3 trials and, if positive, file the new drug application (NDA) around the middle of 2013.
With phase 3 data expected in less than two months, good prior evidence of both safety and efficacy, and a solid cash position, I think Anacor could be an attractive investment at today’s price. The stock is down meaningfully over the past month and investors can buy sizably below the October offering.
SYNTHESIS
References
- Clinical trial number NCT01270971 at ClinicalTrials.gov
- “FDA Approves Anacor Pharmaceuticals’ KERYDIN™ (Tavaborole) Topical Solution, 5% for the Treatment of Onychomycosis of the Toenails”. Market Watch. July 8, 2014.
- http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204427s000lbl.pdf
- http://www.molbase.com/en/hnmr_174671-46-6-moldata-1568017.html#tabs
 |
|
 |
|
| Systematic (IUPAC) name | |
|---|---|
5-Fluoro-2,1-benzoxaborol-1(3H)-ol
|
|
| Clinical data | |
| Trade names | Kerydin |
| Legal status |
|
| Routes of administration |
Topical use only |
| Identifiers | |
| CAS Registry Number | 174671-46-6 |
| ATC code | None |
| PubChem | CID: 11499245 |
| ChemSpider | 9674047 |
| Synonyms | AN2690 |
| Chemical data | |
| Formula | C7H6BFO2 |
| Molecular mass | 151.93 g/mol |
NMR PREDICT
H-NMR spectral analysis
CAS NO. 174671-46-6, 5-fluoro-1-hydroxy-3H-2,1-benzoxaborole H-NMR spectral analysis |
CAS NO. 174671-46-6, 5-fluoro-1-hydroxy-3H-2,1-benzoxaborole C-NMR spectral analysis |
..................................SEE http://newdrugapprovals.org/2013/12/29/tavaborole/
UPDATED………….
http://www.apexbt.com/downloader/document/A3177/NMR.pdf
mp 118-120….http://www.syninnova.com/catalog/product/SL-264
http://www.apexbt.com/downloader/document/A3177/HPLC.pdf
antifugal AN2690 by Anacor
Tavaborole inhibits an essential fungal enzyme, Leucyl-tRNA synthetase, or LeuRS, required for protein synthesis.
Minimum Inhibitory Concentration: 1, 1, 0.5, 0.25, and 0.25 μg/mL for T.rubrum, T.mentagrophytes, C.albicans, C.neoformans, A.fumigatus, respectivley.
AN2690 is a new boron-containing antifungal agent for the potential treatment of onychomycosis. Onychomycosis is caused mainly by dermatophytes, a class of fungus that dwells on skin, hair, and nails and is the cause of other cutaneous fungal infections such as athlete’s foot.
In vitro: AN2690 showed the most active against fungi and especially against the dermatophytes T. rubrum and T. mentagrophytes, the primary fungal pathogens causing onychomycosis. In addition, AN2690 was identified as having a unique profile of in vitro antidermatophyte activity, maintenance of this activity in the presence of keratin, and exceedingly good penetration of human nails [1].
Ex vivo: AN2690 was found to have superior penetration compared to ciclopirox, and achieves levels within and under the nail plate that suggest it has the potential to be an effective topical treatment for onychomycosis [2].
Clinical trial: The efficacy of tavaborole as a topical treatment for onychomycosis has been evaluated in two identical randomised, double-blind phase III studies, NCT01270971 (301) and NCT01302119 (302), enrolling 593 and 601 patients, respectively. Completely or almost clear nail and negative mycology was achieved in 15.3 and 17.9 % of tavaborole recipients compared with 1.5 and 3.9 % of vehicle recipients [3]
References:
[1] Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MR, Sanders V, Plattner JJ. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis. J Med Chem. 2006;49(15):4447-50.
[2] Hui X, Baker SJ, Wester RC, Barbadillo S, Cashmore AK, Sanders V, Hold KM, Akama T, Zhang YK, Plattner JJ, Maibach HI. In Vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96(10):2622-31.
[3] Markham A. Tavaborole: first global approval. Drugs. 2014;74(13):1555-8.
http://www.apexbt.com/downloader/document/A3177/HPLC.pdf
antifugal AN2690 by Anacor
Tavaborole inhibits an essential fungal enzyme, Leucyl-tRNA synthetase, or LeuRS, required for protein synthesis.
Minimum Inhibitory Concentration: 1, 1, 0.5, 0.25, and 0.25 μg/mL for T.rubrum, T.mentagrophytes, C.albicans, C.neoformans, A.fumigatus, respectivley.
AN2690 is a new boron-containing antifungal agent for the potential treatment of onychomycosis. Onychomycosis is caused mainly by dermatophytes, a class of fungus that dwells on skin, hair, and nails and is the cause of other cutaneous fungal infections such as athlete’s foot.
In vitro: AN2690 showed the most active against fungi and especially against the dermatophytes T. rubrum and T. mentagrophytes, the primary fungal pathogens causing onychomycosis. In addition, AN2690 was identified as having a unique profile of in vitro antidermatophyte activity, maintenance of this activity in the presence of keratin, and exceedingly good penetration of human nails [1].
Ex vivo: AN2690 was found to have superior penetration compared to ciclopirox, and achieves levels within and under the nail plate that suggest it has the potential to be an effective topical treatment for onychomycosis [2].
Clinical trial: The efficacy of tavaborole as a topical treatment for onychomycosis has been evaluated in two identical randomised, double-blind phase III studies, NCT01270971 (301) and NCT01302119 (302), enrolling 593 and 601 patients, respectively. Completely or almost clear nail and negative mycology was achieved in 15.3 and 17.9 % of tavaborole recipients compared with 1.5 and 3.9 % of vehicle recipients [3]
References:
[1] Baker SJ, Zhang YK, Akama T, Lau A, Zhou H, Hernandez V, Mao W, Alley MR, Sanders V, Plattner JJ. Discovery of a new boron-containing antifungal agent, 5-fluoro-1,3-dihydro-1-hydroxy-2,1- benzoxaborole (AN2690), for the potential treatment of onychomycosis. J Med Chem. 2006;49(15):4447-50.
[2] Hui X, Baker SJ, Wester RC, Barbadillo S, Cashmore AK, Sanders V, Hold KM, Akama T, Zhang YK, Plattner JJ, Maibach HI. In Vitro penetration of a novel oxaborole antifungal (AN2690) into the human nail plate. J Pharm Sci. 2007;96(10):2622-31.
[3] Markham A. Tavaborole: first global approval. Drugs. 2014;74(13):1555-8.
PAPER
4 1H, 13C, 11B and 19F solid state NMR spectra of AN2690 (red) and BBzx (blue).
Benzoxaboroles are organoboron molecules which are gaining growing interest in different fields, notably for the development of new drugs. However, extensive characterization of these molecules in the solid state is still lacking. Here, questions related to the structure and spectroscopic signatures of crystalline benzoxaborole phases are thus addressed, using a combined experimental-computational approach. Two simple benzoxaboroles were studied: 1,3-dihydro-1-hydroxy-2,1-benzoxaborole (denoted as BBzx) and 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (also referred to as AN2690, a newly developed antifungal drug). First, the crystal structures of AN2690 and BBzx at room temperature are discussed, emphasizing the intermolecular interactions which play an important role in their formation. Then, results of IR and multinuclear (1H, 11B, 13C and 19F) solid state NMR characterization are presented, together with density functional theory (DFT) calculations which were carried out to assist in the interpretation of the spectra. Finally, the influence of polymorphism and anisotropic thermal expansion properties of the crystal structures on the NMR parameters of BBzx and AN2690 is discussed.
A combined experimental-computational study of benzoxaborole crystal structures
*
Corresponding authors
a
Institut Charles Gerhardt de Montpellier, UMR 5253, CNRS-UM2-UM1-ENSCM, Place E. Bataillon, CC1701, 34095 Montpellier cedex 5, France
E-mail: dlaurenc@univ-montp2.fr
Fax: +33 4 67 14 38 58
Tel: +33 4 67 14 38 02
E-mail: dlaurenc@univ-montp2.fr
Fax: +33 4 67 14 38 58
Tel: +33 4 67 14 38 02
b
Sorbonne Universités, UPMC Univ Paris 06, CNRS, Collège de France, UMR 7574, Chimie de la Matière Condensée de Paris, Paris, France
CrystEngComm, 2014,16, 4999-5011
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 LIONEL MY SON
LIONEL MY SON

//////////////
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy
//////////////


