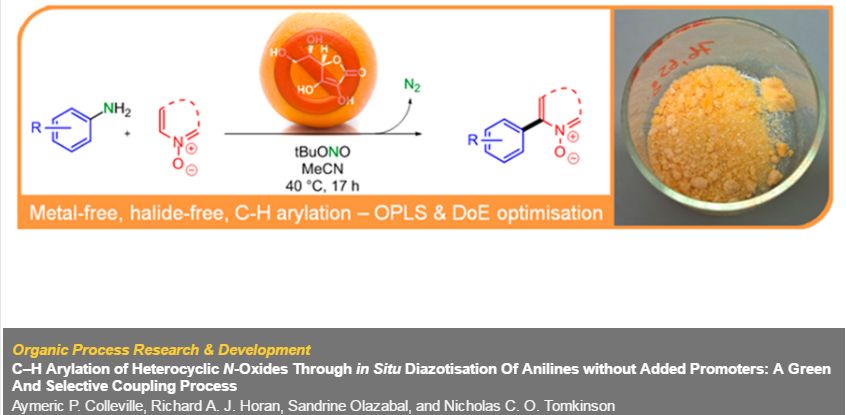
A green and selective method for the generation of biaryl compounds through C–H arylation of heterocyclic N-oxides, in which the addition of ascorbic acid as a promoter is not required for either the generation of an aryldiazonium species or the subsequent arylation, is presented. Reaction conditions were optimized through multivariate data analysis, including orthogonal projections to latent structures (OPLS) and design of experiments (DoE) methodologies, resulting in further sustainability improvements, and were then applied to a range of substrates to establish the scope and limitations of the process. The reaction was studied using in situ infrared spectroscopy and a mechanism is presented that accounts for the available data from this and previous studies. The reaction was also performed on a multigram scale, with calorimetry studies to support further scale-up of this promoter-free transformation.
C–H Arylation of Heterocyclic N-Oxides Through in Situ Diazotisation Of Anilines without Added Promoters: A Green And Selective Coupling Process
† API Chemistry, GlaxoSmithKline Research and Development Ltd., Gunnels Wood Road, Stevenage, Hertfordshire SG1 2NY, U.K.
‡ WestCHEM, Department of Pure and Applied Chemistry, Thomas Graham Building, University of Strathclyde, 295 Cathedral Street, Glasgow G1 1XL, U.K.
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00117
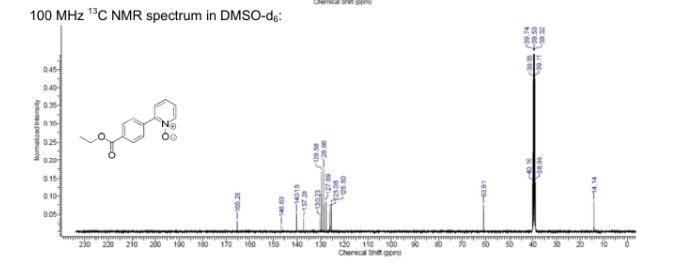
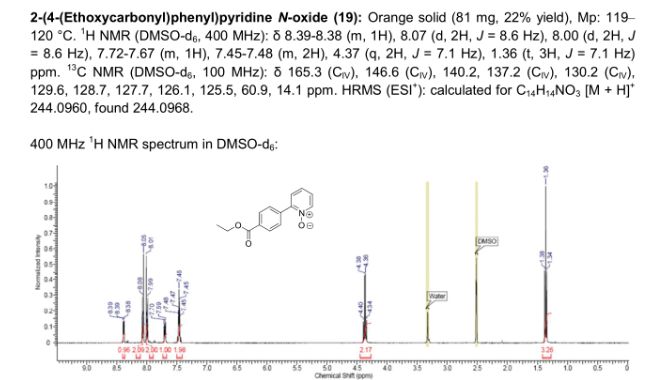
2-(4-(Ethoxycarbonyl)phenyl)pyridine N-Oxide
Orange solid (81 mg, 22% yield), mp 119–120 °C.
1H NMR (DMSO-d6, 400 MHz): δ 8.39–8.38 (m, 1H), 8.07 (d, 2H, J = 8.6 Hz), 8.00 (d, 2H, J = 8.6 Hz), 7.72–7.67 (m, 1H), 7.45–7.48 (m, 2H), 4.37 (q, 2H, J = 7.1 Hz), 1.36 (t, 3H, J = 7.1 Hz) ppm.
13C NMR (DMSO-d6, 100 MHz): δ 165.3 (CIV), 146.6 (CIV), 140.2, 137.2 (CIV), 130.2 (CIV), 129.6, 128.7, 127.7, 126.1, 125.5, 60.9, 14.1 ppm.
HRMS (ESI+): calculated for C14H14NO3 [M+H]+ 244.0960, found 244.0968.
//////C–H Arylation of Heterocyclic N-Oxides, Situ Diazotisation Of Anilines, Promoters, Green And Selective Coupling Process