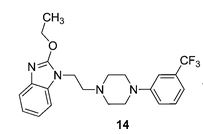
2-Ethoxy-1-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethyl)-1H-benzo[d]imidazole
SCHEME

2-Ethoxy-1-(2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethyl)-1H-benzo[d]imidazole (14)
A reactor was charged with compound 13 (500 g, 2.23 mol), compound 10 (595 g, 2.23 mol), sodium iodide (334 g, 2.23 mol), potassium carbonate (338 g, 2.45 mol), and water (3 L). Then the mixture was heated at reflux for 12 h. The reaction mixture was cooled to room temperature and extracted with ethyl acetate (2 L). The organic layer was washed with water (700 mL) and brine (500 mL), dried, and evaporated under reduced pressure to provide 14 which was used directly in the next step (857 g, 92%).
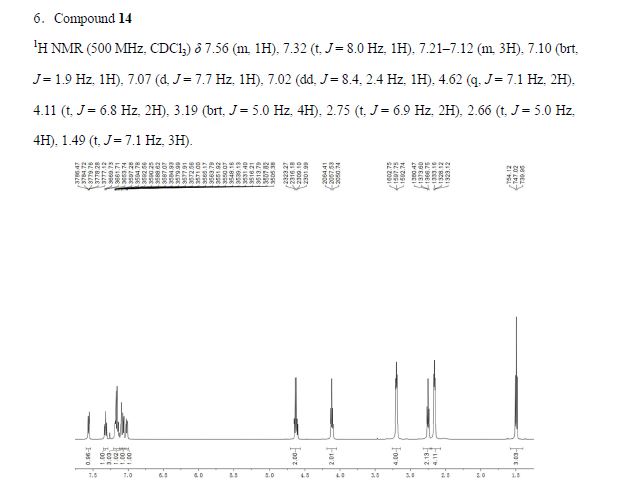
1H NMR (500 MHz, CDCl3) δ 7.56 (m, 1H), 7.32 (t, J = 8.0 Hz, 1H), 7.21–7.12 (m, 3H), 7.10 (brt, J = 1.9 Hz, 1H), 7.07 (d, J = 7.7 Hz, 1H), 7.02 (dd, J = 8.4, 2.4 Hz, 1H), 4.62 (q, J = 7.1 Hz, 2H), 4.11 (t, J = 6.8 Hz, 2H), 3.19 (brt, J = 5.0 Hz, 4H), 2.75 (t, J = 6.9 Hz, 2H), 2.66 (t, J = 5.0 Hz, 4H), 1.49 (t, J = 7.1 Hz, 3H).
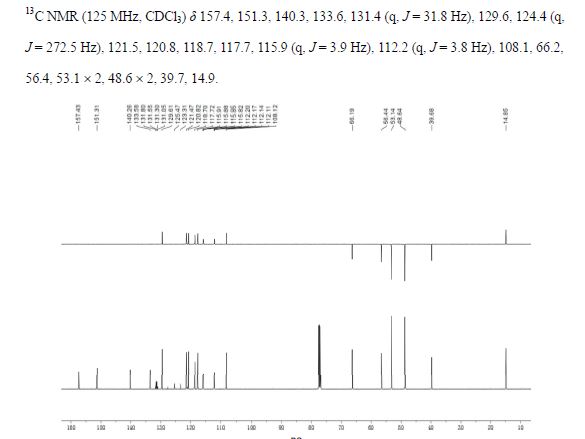
13C NMR (125 MHz, CDCl3) δ 157.4, 151.3, 140.3, 133.6, 131.4 (q, J = 31.8 Hz), 129.6, 124.4 (q, J = 272.5 Hz), 121.5, 120.8, 118.7, 117.7, 115.9 (q, J = 3.9 Hz), 112.2 (q, J = 3.8 Hz), 108.1, 66.2, 56.4, 53.1 × 2, 48.6 × 2, 39.7, 14.9.
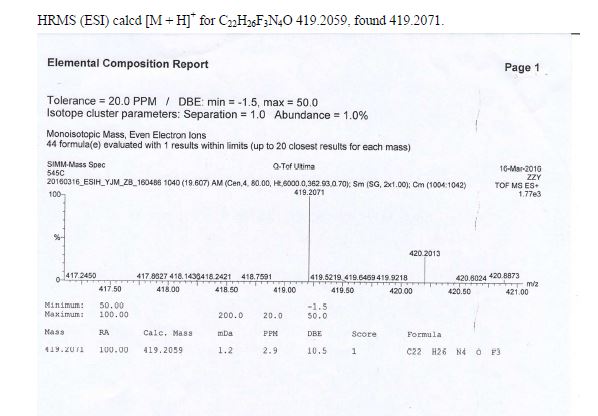
ESI-MS (m/z): 419.6 [M + H]+; HRMS (ESI) calcd [M + H]+ for C22H26F3N4O 419.2059, found 419.2071.
HPLC: retention time of 12.4 min, 95% purity.