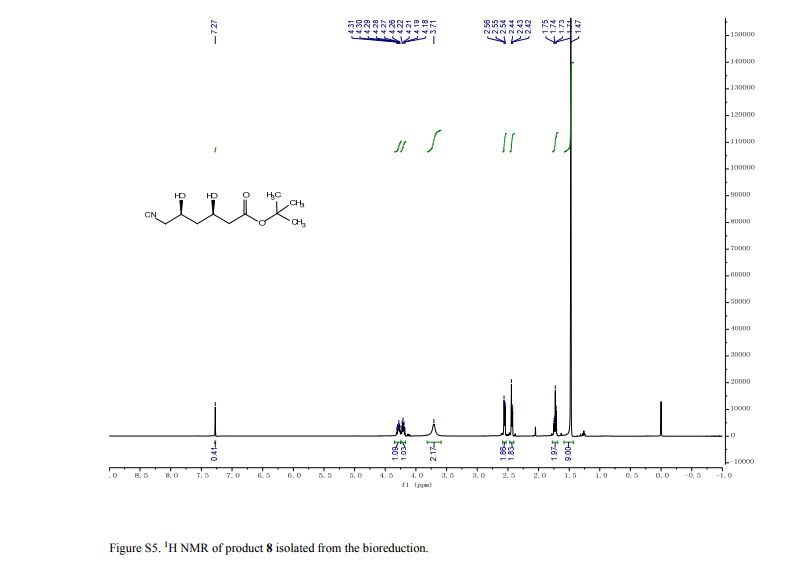
pure 8 (2.4 g, HPLC purity, 92%). [α]D25 = −41.3 (c = 1.0, CHCl3), >99.5% de.(8g, 9) 1H NMR (CDCl3, 400 MHz), δ/ppm: 1.47 (s, 9H), 1.71–1.75 (m, 2H), 2.42–2.44 (m, 2H), 2.54–2.56 (m, 2H), 3.71 (brs, 2H), 4.18–4.22 (m, 1H), 4.26–4.31 (m, 1H).
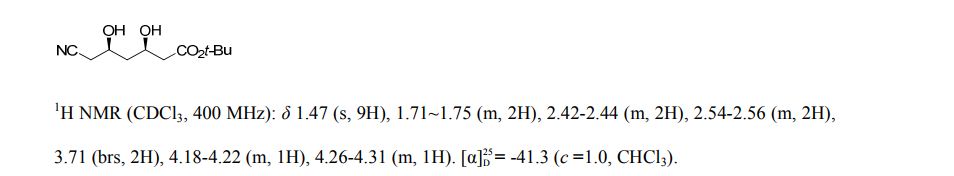
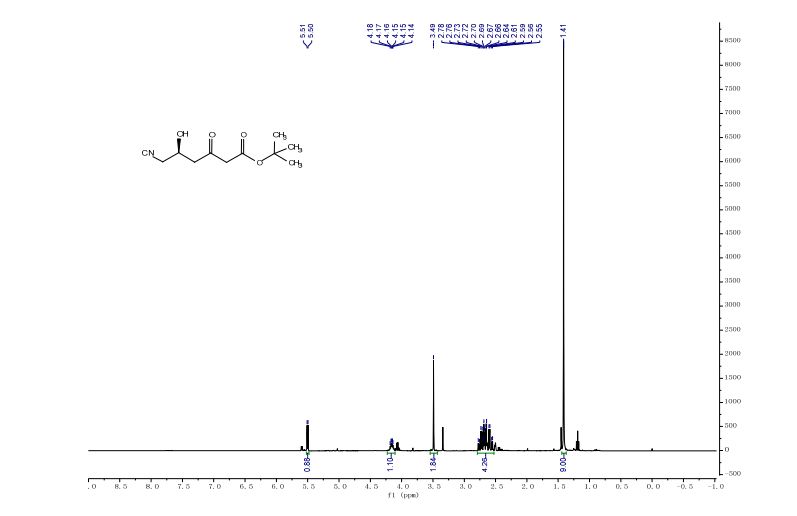
t-butyl 6-cyano-(5R)-hydroxy-3-oxo-hexanoate


t-Butyl-6-cyano-(3R,5R)-dihydroxyhexanoate is an advanced chiral precursor for the synthesis of the side chain pharmacophore of cholesterol-lowering drug atorvastatin. Herein, a robust carbonyl reductase (LbCR) was newly identified from Lactobacillus brevis, which displays high activity and excellent diastereoselectivity toward bulky t-butyl 6-cyano-(5R)-hydroxy-3-oxo-hexanoate (7). The engineered Escherichia coli cells harboring LbCR and glucose dehydrogenase (for cofactor regeneration) were employed as biocatalysts for the asymmetric reduction of substrate 7. As a result, as much as 300 g L–1 of water-insoluble substrate was completely converted to the corresponding chiral diol with >99.5% de in a space–time yield of 351 g L–1 d–1, indicating a great potential of LbCR for practical synthesis of the very bulky and bi-chiral 3,5-dihydroxy carboxylate side chain of best-selling statin drugs.
Identification of a Robust Carbonyl Reductase for Diastereoselectively Building syn-3,5-Dihydroxy Hexanoate: a Bulky Side Chain of Atorvastatin
† State Key Laboratory of Bioreactor Engineering, Shanghai Collaborative Innovation Center for Biomanufacturing, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China
‡ School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, P. R. China
§ Guangxi Key Laboratory of Biorefinery, Guangxi Academy of Sciences, Nanning 530003, Guangxi, P. R. China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00194
*E-mail: gaoweizheng@ecust.edu.cn; fax: (+86)-21-64250840., *E-mail: jianhexu@ecust.edu.cn; fax: (+86)-21-64252498.
