
.
 1
1H NMR (200 MHz, CDCl
3):4.12 (bs, 1H), 4.66 (s, 2H), 6.49 (d,
J=4Hz, 1H), 7.21 (d,
J=4Hz, 1H), 9.49 (s, 1H).
 FT IR HPLC
FT IR HPLC
 1H NMR
1H NMR
 13C NMR
Hydroxymethylfurfural
13C NMR
Hydroxymethylfurfural (HMF), also 5-(Hydroxymethyl)furfural, is an
organic compound derived from dehydration of certain
sugars.
[2][3][4] This yellow low-melting solid is highly water-soluble. The molecule consists of a
furan ring, containing both
aldehyde and
alcohol functional groups. HMF has been identified in a wide variety of baked goods. HMF, which is derived from
hexoses, is a potential "
carbon-neutral" feedstock for fuels and chemicals.
[5]
Production and reactions
Related to the production of
furfural, HMF is produced from
sugars. It arises via the dehydration of
fructose.
[6] Treatment of fructose with acids followed by
liquid-liquid extraction into organic solvents such as
methyl isobutyl ketone. The conversion is affected by various additives such as
DMSO,
2-butanol, and
polyvinyl pyrrolidone, which minimize the formation of
side product. Ionic liquids facilitate the conversion of fructose to HMF.
[7] When hexoses are hydrolyzed with
hydrochloric acid,
5-chloromethylfurfural is produced instead of HMF.

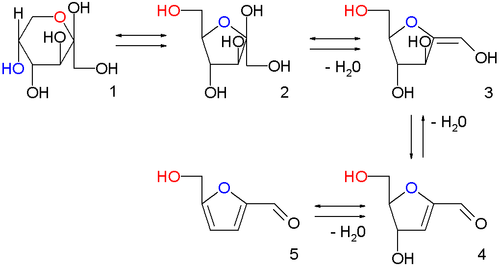
In the image above are displayed in a series of
chemical equilibria: fructopyranose
1, fructofuranose
2, two intermediate stages of
dehydration (not isolated)
3,4 and finally HMF
5.
Chromous chloride catalyzes the direct conversion of both
fructose (yielding 90%+) and
glucose (yielding 70%+) into an HMF.
[8]
Cellulose can also be converted into HMF (yielding 55% at 96% purity),
in a process that proceeds via the intermediacy of glucose and fructose.
[9][10]
HMF can be converted to
2,5-dimethylfuran (DMF), a liquid that is a potential biofuel with a greater energy content than
bioethanol.
Oxidation of HMF gives
2,5-furandicarboxylic acid, which has been proposed as a replacement for
terephthalic acid in the production of polyesters. Reduction gives
2,5-bis(hydroxymethyl)furan. Acid-catalysed hydrolysis converts HMF into
gamma-valerolactone, with loss of formic acid.
[11]
Occurrence in food
HMF is practically absent in fresh food, but it is naturally
generated in sugar-containing food during heat-treatments like drying or
cooking. Along with many other flavor- and color-related substances,
HMF is formed in the
Maillard reaction as well as during
caramelization. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF.
[12]
HMF is a well known component of baked goods. Upon toasting bread, the
amount increases from 14.8 (5 min.) to 2024.8 mg/kg (60 min).
[3]
It is a good
wine storage time−temperature marker,
[13] especially in
sweet wines such as
Madeira[14] and those sweetened with grape concentrate
arrope.
[15]
As an unwanted component
HMF can be found in low amounts in
honey, fruit-
juices and
UHT-milk.
Here, as well as in vinegars, jams, alcoholic products or biscuits HMF
can be used as an indicator for excess heat-treatment. For instance,
fresh honey contains less than 15 mg/kg—depending on pH-value and
temperature and age,
[16] and the
codex alimentarius standard
requires that honey have less than 40 mg/kg HMf to guarantee that the
honey has not undergone heating during processing, except for tropical
honeys which must be below 80 mg/kg.
Higher quantities of HMF are found naturally in coffee and dried
fruit. Several types of roasted coffee contained between 300 –
2900 mg/kg HMF.
[17] Dried plums were found to contain up to 2200 mg/kg HMF. In dark beer 13.3 mg/kg were found,
[18] bakery-products contained between 4.1 – 151 mg/kg HMF.
[19]
It can be found in
glucose syrup.
HMF can form in
high-fructose corn syrup (HFCS), levels around 20 mg/kg HMF were found, increasing during storage or heating.
[16] This is a problem for American
beekeepers because they use HFCS as a source of sugar when there are not enough
nectar sources to feed
honeybees, and HMF is toxic to them. Adding bases such as soda ash or potash to
neutralize the HFCS slows the formation of HMF.
[16]
Depending on production-technology and storage, levels in food vary
considerably. To evaluate the contribution of a food to HMF intake, its
consumption-pattern has to be considered. Coffee is the food that has a
very high relevance in terms of levels of HMF and quantities consumed.
HMF is a natural component in heated food but usually present in low
concentrations. The daily intake of HMF may underlie high variations due
to individual consumption-patterns. It has been estimated that in a
western diet, in the order of magnitude of 5 – 10 mg of HMF are ingested
per day from food.
[12]
In former times, HMF was used in food for flavoring purposes, but in
Europe this practice now is suspended. HMF is also found in cigarette
smoke.
[20]
Biomedical
A major
metabolite
in humans is 5-hydroxymethyl-2-furoic acid (HMFA), which is excreted in
urine. HMF can also be metabolized to 5-sulfoxymethylfurfural (SMF),
which is highly reactive and can form adducts with DNA or proteins. In
vitro tests and studies on rats suggest potential toxicity and
carcinogenicity of HMF.
[21] In humans, no correlation between intakes of HMF and disease is known.
HMF has been found to bind specifically with intracellular sickle hemoglobin (HbS). Preliminary
in vivo studies using transgenic sickle mice showed that orally administered 5HMF inhibits the formation of sickled cells in the blood.
[22] Under the development code Aes-103, HMF has been considered for the treatment of
sickle cell disease.
[23]
Quantification
Today,
HPLC with UV-detection is the reference-method (e.g. DIN 10751-3). Classic methods for the quantification of HMF in food use
photometry. The method according to White is a differential UV-photometry with and without sodium bisulphite-reduction of HMF (
AOAC 980.23). Winkler photometric method is a colour-reaction using p-
toluidine and
barbituric acid (
DIN
10751-1). Photometric test may be unspecific as they may detect also
related substances, leading to higher results than HPLC-measurements.
Test-kits for rapid analyses are also available (e.g. Refelctoquant HMF,
Merck KGaA).
[24][25]
History
This
organic compound was first prepared from
inulin using
oxalic acid.
[26] It was examined by
French chemist Louis Maillard in 1912 in studies on non-enzymatic reactions of
glucose.
Its conversion to myriad organic compounds, e.g., solvents, polymer
precursors, and biofuels has been regularly studied since the 1950's. In
the 1980s, the role of acids in its formation was elucidated,
especially means of avoiding the formation of
humins.
[3]
Other
HMF is an intermediate in the titration of hexoses in the
Molisch's test. In the related
Bial's test
for pentoses, the hydroxymethylfurfural from hexoses may give a
muddy-brown or gray solution, but this is easily distinguishable from
the green color of pentoses.
References
The
Determination of HMF in Honey with an Evolution Array UV-Visible
Spectrophotometer. Nicole Kreuziger Keppy and Michael W. Allen, Ph.D.,
Application note 51864, Thermo Fisher Scientific, Madison, WI, USA (article)
Malgorzata
E. Zakrzewska, Ewa Bogel-Lukasik, Rafal Bogel-Lukasik "Ionic
Liquid-Mediated Formation of 5-Hydroxymethylfurfurals-A Promising
Biomass-Derived Building Block" Chem. Rev., 2011, volume 111, 397. doi:10.1021/cr100171a
Andreia
A. Rosatella, Svilen P. Simeonov, Raquel F. M. Frade, Carlos A. M.
Afonso "Critical Review 5-Hydroxymethylfurfural (HMF) as a building
block platform: Biological Properties, Synthesis and Synthetic
Applications" Green Chem., 2011, volume 13, 754. doi:10.1039/c0gc00401d
Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources
Robert-Jan van Putten, Jan C. van der Waal, Ed de Jong, Carolus B.
Rasrendra, Hero J. Heeres, and Johannes G. de Vries Chemical Reviews
2013, vol. 113, pp. 1499–1597. doi:10.1021/cr300182k
Huber, George W.; Iborra, Sara; Corma, Avelino (2006). "Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering". Chem. Rev. 106 (9): 4044–98. doi:10.1021/cr068360d. PMID 16967928.MIT Technology Review
Yuriy
Román-Leshkov, Juben N. Chheda, James A. Dumesic (2006). "Phase
Modifiers Promote Efficient Production of Hydroxymethylfurfural from
Fructose". Science 312 (5782): 1933–1937. doi:10.1126/science.1126337. PMID 16809536.
Ståhlberg,
T.; Fu, W.; Woodley, J. M.; Riisager, A. "Synthesis of
5-(Hydroxymethyl)furfural in Ionic Liquids: Paving the Way to Renewable
Chemicals" ChemSusChem. 2011, Volume 4, pages 451–458. doi:10.1002/cssc.201000374
Haibo
Zhao, Johnathan E. Holladay, Heather Brown, Z. Conrad Zhang (2007).
"Metal Chlorides in Ionic Liquid Solvents Convert Sugars to
5-Hydroxymethylfurfural". Science 316 (5782): 1597–1600. doi:10.1126/science.1141199. PMID 17569858.
Su,
Yu; Brown, Heather M.; Huang, Xiwen; Zhou, Xiao-Dong; Amonette, James
E.; Zhang, Z. Conrad (2009). "Single-step conversion of cellulose to
5-hydroxymethylfurfural (HMF), a versatile platform chemical". Applied Catalysis A: General 361: 117. doi:10.1016/j.apcata.2009.04.002.
A.
A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso,
"5-Hydroxymethylfurfural (HMF) as a building block platform: Biological
properties, synthesis and synthetic applications", Green Chemistry 2011,
vol. 13, 754-793. doi:10.1039/c0gc00401d
van
Putten, R.-J., van der Waal, J. C., de Jong, E., Rasrendra, C. B.,
Heeres, H. J.,de Vries, J. G., "Hydroxymethylfurfural, A Versatile
Platform Chemical Made from Renewable Resources", Chem. Rev. 2013, 113,
1499.
Arribas-Lorenzo,
G; Morales, FJ (2010). "Estimation of dietary intake of
5-hydroxymethylfurfural and related substances from coffee to Spanish
population". Food and Chemical Toxicology 48 (2): 644–9. doi:10.1016/j.fct.2009.11.046. PMID 20005914.
Kinetics
of Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial
Sparkling Wines. A. Serra-Cayuela, M. Jourdes, M. Riu-Aumatell, S.
Buxaderas, P.-L. Teissedre and E. López-Tamames, J. Agric. Food Chem.,
2014, volume 62, issue 5, pages 1159–1166, doi:10.1021/jf403281y
Evolution
of 5-hydroxymethylfurfural (HMF) and furfural (F) in fortified wines
submitted to overheating conditions. V. Pereira, F.M. Albuquerque, A.C.
Ferreira, J. Cacho and J.C. Marques, Food Research International, Volume
44, Issue 1, January 2011, Pages 71–76, doi:10.1016/j.foodres.2010.11.011
Hydroxymethylfurfural
in California wines. Maynard A. Amerine, Journal of Food Science, May
1948, Volume 13, Issue 3, pages 264–269, doi:10.1111/j.1365-2621.1948.tb16621.x
Ruiz-Matute,
AI; Weiss, M; Sammataro, D; Finely, J; Sanz, ML (2010). "Carbohydrate
composition of high-fructose corn syrups (HFCS) used for bee feeding:
effect on honey composition". Journal of Agricultural and Food Chemistry 58 (12): 7317–22. doi:10.1021/jf100758x. PMID 20491475.
Murkovic, M; Pichler, N (2006). "Analysis of 5-hydroxymethylfurfual in coffee, dried fruits and urine". Molecular Nutrition & Food Research 50 (9): 842–6. doi:10.1002/mnfr.200500262. PMID 16917810.
Husøy,
T; Haugen, M; Murkovic, M; Jöbstl, D; Stølen, LH; Bjellaas, T;
Rønningborg, C; Glatt, H; Alexander, J (2008). "Dietary exposure to
5-hydroxymethylfurfural from Norwegian food and correlations with urine
metabolites of short-term exposure". Food and Chemical Toxicology 46 (12): 3697–702. doi:10.1016/j.fct.2008.09.048. PMID 18929614.
Ramírez-Jiménez,
A; Garcı́a-Villanova, Belén; Guerra-Hernández, Eduardo (2000).
"Hydroxymethylfurfural and methylfurfural content of selected bakery
products". Food Research International 33 (10): 833. doi:10.1016/S0963-9969(00)00102-2.
Rufían-Henares, JA; De La Cueva, SP (2008). "Assessment of hydroxymethylfurfural intake in the Spanish diet". Food Additives & Contaminants: Part A: Chemistry, Analysis, Control, Exposure & Risk Assessment 25 (11): 1306–12. doi:10.1080/02652030802163406. PMID 19680837.
Husøy,
T.; Haugen, M.; Murkovic, M.; Jöbstl, D.; Stølen, L.H.; Bjellaas, T.;
Rønningborg, C.; Glatt, H.; Alexander, J. (2008). "Dietary exposure to
5-hydroxymethylfurfural from Norwegian food and correlations with urine
metabolites of short-term exposure". Food and Chemical Toxicology 46 (12): 3697–702. doi:10.1016/j.fct.2008.09.048. PMID 18929614.
Abdulmalik,
O; Safo, MK; Chen, Q; Yang, J; Brugnara, C; Ohene-Frempong, K; Abraham,
DJ; Asakura, T (2005). "5-hydroxymethyl-2-furfural modifies
intracellular sickle haemoglobin and inhibits sickling of red blood
cells". British journal of haematology 128 (4): 552–61. doi:10.1111/j.1365-2141.2004.05332.x. PMID 15686467.
"Aes-103 Drug Development". AesRx.
Schultheiss,
J.; Jensen, D.; Galensa, R. (2000). "Determination of aldehydes in food
by high-performance liquid chromatography with biosensor coupling and
micromembrane suppressors". Journal of Chromatography A 880: 233. doi:10.1016/S0021-9673(99)01086-9.
Gaspar,
Elvira M.S.M.; Lucena, Ana F.F. (2009). "Improved HPLC methodology for
food control – furfurals and patulin as markers of quality". Food Chemistry 114 (4): 1576. doi:10.1016/j.foodchem.2008.11.097.
- G. Dull, Chemiker Zeitung, 1895, 216.


 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
Join me on Linkedin

Join me on Facebook
 FACEBOOK
FACEBOOK
Join me on twitter

 amcrasto@gmail.com
amcrasto@gmail.com
////////////



