A practical and scalable synthesis of halo quinolin-2(1H)-ones is presented. The heterocycles are easily accessed from inexpensive halo anilines in a two-step sequence. The anilines are acylated with methyl 3,3-dimethoxypropionate under basic conditions in quantitative yields. The crude amides undergo cyclization in sulfuric acid to the desired halo quinolin-2(1H)-ones in 28–93% yield (2 steps). The synthetic sequence was successfully applied on 800 g scale. Anilines with strong electron withdrawing or electron donating groups were poor substrates for this procedure. 6-Iodoquinolin-2(1H)-one and 6-bromo-8-iodoquinolin-2(1H)-one were further functionalized to obtain quinolines substituted with various functional groups.
Scalable and Practical Synthesis of Halo Quinolin-2(1H)-ones and Quinolines
Chemistry Process R&D, Actelion Pharmaceuticals Ltd., Gewerbestrasse 16, CH-4123 Allschwil, Switzerland
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00124
*E-mail: Gunther.Schmidt@idorsia.com. Telephone: +41 61 565 8028.
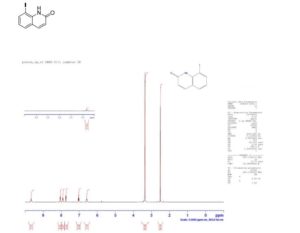
1H NMR spectrum of 8-iodoquinolin-2(1H)-one 3a (in D6-DMSO).
8-Bromo-2-quinolin-2(1H)-one (3b). Obtained from amide 2b according to the general cyclisation procedure A as yellow solid. Yield: 8.0 g (89%). Purity (LC-MS): 99.3% (210 nm), tR 0.69 min;
[M + 1]+ = 226,
mp 183 °C (DSC);
1H NMR (D6-DMSO) δ: 10.51 (s, 1 H), 7.96 (d, J = 9.5 Hz, 1 H), 7.83 (m, 1 H), 7.73 (m, 1 H), 7.16 (m, 1 H), 6.61 (d, J = 9.5 Hz, 1 H);
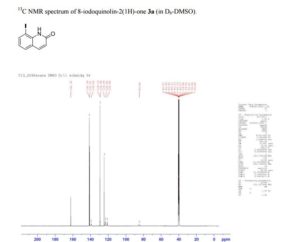
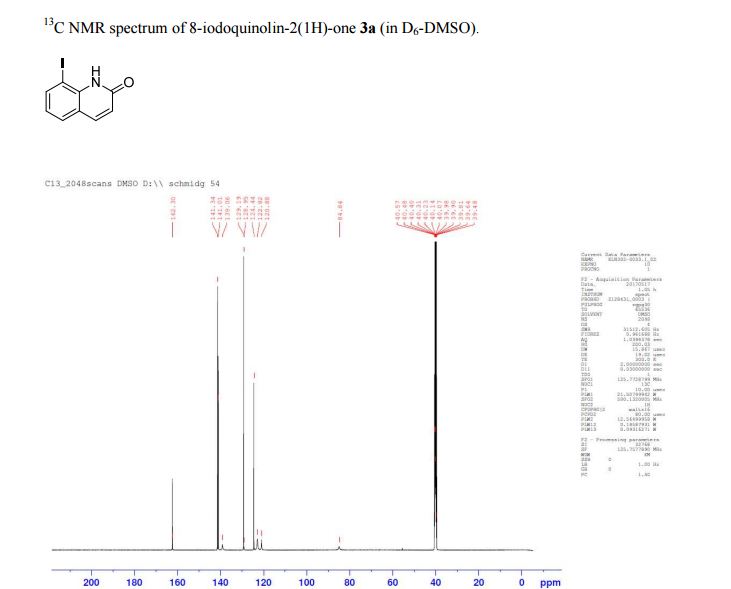
13C NMR (D6-DMSO) δ: 162.3, 141.0, 136.7, 134.4, 128.5, 123.6, 123.2, 121.5, 108.5

/////////