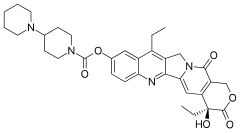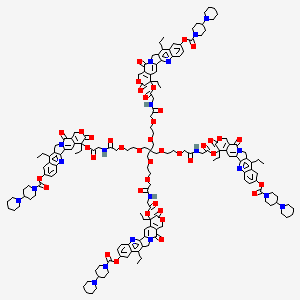

Etirinotecan pegol (NKTR-102)
848779-32-8
PEG-irinotecan
Also known as: NKTR-102; UNII-LJ16641SFT; 848779-32-8
Molecular Formula: C161H192N20O40 Molecular Weight: 3047.35718

CAS: 1193151-09-5
Synonym: NKTR102; NKTR 102; NKTR-102; pegylated irinotecan NKTR 102; Etirinotecan pegol.
IUPAC/Chemical name: (1). Tetrakis{(4S)-9-[([1,4'-bipiperidinyl]-1'-carbonyl)oxy]-4,11-diethyl-3,14-dioxo-3,4,12,14- tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-4-yl} N,N',N'',N'''- {methanetetrayltetrakis[methylenepoly(oxyethylene)oxy(1-oxoethylene)]}tetraglycinate tetrahydrochloride
(2). Poly(oxy-1,2-ethanediyl), α-hydro-ω-[2-[[2-[[(4S)-9-[([1,4'-bipiperidin]-1'-ylcarbonyl)oxy]- 4,11-diethyl-3,4,12,14-tetrahydro-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2- b]quinolin-4-yl]oxy]-2-oxoethyl]amino]-2-oxoethoxy]-, ether with 2,2-bis(hydroxymethyl)- 1,3-propanediol, hydrochloride (4:1:4)
Etirinotecan pegol tetratriflutate [USAN]
RN: 1193151-12-0
Tetrakis((4S)-9-(((1,4'-bipiperidinyl)-1'-carbonyl)oxy)-4,11-diethyl-3,14-dioxo-3,4,12,14- tetrahydro-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinolin-4-yl) N,N',N'',N'''- (methanetetrayltetrakis(methylenepoly(oxyethylene)oxy(1-oxoethylene)))tetraglycinate tetrakis(trifluoroacetate)
NKTR-102 is currently being developed by Nektar.
According to the company's news release, this agent exhibits a very high response rate and excellent clinical benefit rate in patients with metastatic breast cancer, and importantly, this anti-tumor activity is maintained in each of the poor prognosis subsets within the study. The data from the Phase 2 study also shows highly promising PFS of 5.3 months and OS of 13.1 months in the every three week dose schedule, which was also very well-tolerated. As a novel topoisomerase I inhibitor in breast cancer, NKTR-102 holds great therapeutic potential and allows us to address the challenge of resistance in this setting
NKTR-102 (PEG-irinotecan), a PEGylated form of irinotecan, is in clinical development by Nektar Therapeutics for the treatment of multiple solid tumors, including colorectal cancer, metastatic or locally advanced breast cancer, metastatic or locally advanced ovarian cancer and gastrointestinal cancer. No recent development has been reported for phase I clinical trials for the treatment of gastrointestinal cancer.
In preclinical studies, NKTR-102 resulted in significantly higher reduction in tumor growth than irinotecan in colon, lung and breast tumors. The company believes that following intravenous administration of NKTR-102, irinotecan will be released slowly, resulting in prolonged systemic exposure of irinotecan. Irinotecan is a cytotoxic anticancer agent used extensively to treat colorectal, lung, esophageal and other solid tumors. In 2011, orphan drug designation was assigned to the compound in the U.S. for the treatment of ovarian cancer.
In 2011, orphan drug designation was assigned in the E.U. for the treatment of ovarian cancer. In 2012, fast track designation was assigned by the FDA for the treatment of locally recurrent or metastatic breast cancer progressing after treatment with an anthracycline, a taxane and capecitabine.
| Therapeutic Area | Nektar
Discovered | Indication | Phase |
| Oncology | |
| Etirinotecan pegol (NKTR-102) |
 | Metastatic Breast Cancer | 
Phase 3 |
 | Platinum-Resistant Ovarian Cancer | 
Phase 2 Completed |
 | Second-Line Colorectal Cancer | 
Phase 2 Completed |
 | Bevacizumab (Avastin)-refractory high-grade glioma | 
Phase 2 |
 | Non-Small Cell Lung Cancer (NSCLC) | 
Phase 2 |
 | Small Cell Lung Cancer (SCLC) | 
Phase 2 |
 | GI and solid tumors
In combination with 5-FU | 
Phase 1 Completed |
 |
Market Overview
Etirinotecan pegol is in Phase 3 clinical development for patients with metastatic or locally recurrent breast cancer and Phase 2 clinical development for patients with solid tumor malignancies, including ovarian, colorectal, glioma, small cell and non-small cell lung cancers. Each year, approximately 5.3 million patients worldwide are diagnosed with one of these types of cancer.1
Etirinotecan Pegol Clinical Data and Product Profile
Etirinotecan pegol (NKTR-102) is the first long-acting topoisomerase I-inhibitor (Topo I) designed to concentrate in tumor tissue, provide sustained tumor suppression throughout the entire chemotherapy cycle, and to reduce the peak exposures that are associated with toxicities of other cytotoxics. Etirinotecan pegol was invented by Nektar using its advanced polymer conjugate technology platform, and is the first oncology product candidate to leverage Nektar's releasable polymer technology platform.
Topo I-inhibitors are important chemotherapeutic agents used to treat cancer. Immediately after dosing, however, standard topo I-inhibitors reach high peak concentrations and diffuse quickly throughout the body---penetrating and damaging healthy tissue, such as bone marrow, as well as tumor tissue. Subsequent rapid metabolism limits topo I exposure in tumor cells, reducing the duration of their effect and resulting in a much lower tumor exposure to the active metabolite that may limit their efficacy.
Etirinotecan pegol is a novel chemotherapeutic designed to enhance the anti-cancer effects of topo I-inhibition while minimizing its toxicities. Unlike first generation topo I-inhibitors that exhibit a high initial peak concentration and short half-life, etirinotecan pegol's unique pro-drug design results in a lowered initial peak concentration of active topo I inhibitor in the blood. The large etirinotecan pegol molecule is inactive when administered. Over time, the body's natural enzymatic processes slowly metabolize the linkers within the molecule, continuously freeing active drug that then works to stop tumor cell division through inhibition of topo I.
Clinical and preclinical studies have shown that the half-life of active drug generated from etirinotecan pegol is greatly extended to 50 days (compared to 48 hours for irinotecan) and that active drug remains in circulation throughout the entire chemotherapy cycle, providing sustained exposure to topo I inhibition. In preclinical models, etirinotecan pegol achieved a 300-fold increase in tumor concentration as compared to a first generation topo I-inhibitor. Because etirinotecan pegol is a large molecule, it is believed to penetrate the leaky vasculature within the tumor environment more readily than normal vasculature, concentrating and trapping etirinotecan pegol in tumor tissue.
Etirinotecan pegol is currently in development for the treatment of breast, ovarian, colorectal, glioma, small cell and non-small cell lung cancers.
Ongoing clinical development for etirinotecan pegol:
- In metastatic breast cancer, a Phase 3 randomized, head to head study (The BEACON Study) of etirinotecan pegol compared to Treatment of Physician's Choice (TPC) completed enrollment of 864 patients in August 2013. Data from the study on the primary endpoint of overall survival is expected by the end of 2014 or early 2015.
- In ovarian cancer, an expanded Phase 2 study of single-agent etirinotecan pegol in platinum refractory/resistant ovarian cancer in 177 women who failed prior Doxil therapy was completed at the end of 2012.
- In colorectal cancer, a 174-patient Phase 2 randomized, head-to-head study of etirinotecan pegol compared to irinotecan in patients with second-line colorectal cancer with the KRAS gene mutation is in progress.
- Etirinotecan pegol is also being evaluated in glioma, small cell and non-small cell lung cancers.
Highlighted Data Presentations:
Data from a Phase 2 clinical study of etirinotecan pegol in metastatic breast cancer were presented in an oral abstract session at the 2011 ASCO Breast Cancer Symposium by Agustin Garcia, MD.
View presentation slides.
Data from a Phase 2 clinical study of NKTR-102 in a subpopulation of patients with platinum-resistant/refractory ovarian cancer and prior Doxil® (pegylated liposomal doxorubicin or PLD) treatment were presented at the 2011 ASCO Annual Meeting by Agustin Garcia, MD. (
click here to download this presentation).
Data from a Phase 2 clinical study of etirinotecan pegol in metastatic breast cancer were presented at the 2010 33rd Annual CTRC-AACR San Antonio Breast Cancer Symposium by Amad Awada, MD. (
click here to download this presentation).
| January 16-18, 2014 | 2014 Gastrointestinal Cancers SymposiumPoster C55: "A phase I study of etirinotecan pegol in combination with 5-fluorouracil and leucovorin in patients with advanced cancer." January 18, 2014 | San Francisco, CA |
| February 22, 2014 | 26.2 with Donna Marathon sponsored by Mayo Clinic | Jacksonville, FL |
| March 5-7, 2014 | TAT 2013: International Congress on Targeted Anticancer Therapies | Washington, DC |
| April 5-9, 2014 | AACR Annual Meeting 2013 | San Diego, CA |
| May 19-21, 2014 | 10th International Symposium on Polymer Therapeutics | Valencia, Spain |
| May 30-June 3, 2014 | 2014 ASCO 50th Annual MeetingPoster Presentation: "Combination Immunotherapy: Synergy of a Long-Acting Engineered Cytokine (NKTR-214) and Checkpoint Inhibitors Anti-CTLA-2 or Anti-PD-1 in Murine Tumor Models," Kantak et al.
Abstract Number: 3082
Session Title/Track: Developmental Therapies - Immunotherapy
Date: June 1, 2014, 8:00 a.m. - 11:45 a.m. Central Time | Chicago, Illinois |
| September 4-6, 2014 | ASCO Breast Cancer Symposium | San Francisco, CA |
| September 26-30, 2014 | ESMO 2014 Congress | Madrid, Spain |
| December 9-13, 2014 | San Antonio Breast Cancer Symposium | San Antonio, TX |
............................
Example 1 SYNTHESIS OF PENTAERYTHRITOLYL-4-ARM-(PEG-1-METHYLENE-2 OXO-VINYLAMINO ACETATE LINKED-IRINOTECAN)-20K
A. Synthesis of t-Boc-Glycine-Irinotecan
In a flask, 0.1 g Irinotecan (0.1704 mmoles), 0.059 g t-Boc-Glycine (0.3408 mmoles), and 0.021 g DMAP (0.1704 mmoles) were dissolved in 13 mL of anhydrous dichloromethane (DCM). To the solution was added 0.070 g DCC (0.3408 mmoles) dissolved in 2 mL of anhydrous DCM. The solution was stirred overnight at room temperature. The solid was removed through a coarse frit, and the solution was washed with 10 mL of 0.1N HCL in a separatory funnel. The organic phase was further washed with 10 mL of deionized H2O in a separatory funnel and then dried with Na2SO4. The solvent was removed using rotary evaporation and the product was further dried under vacuum. 1H NMR (DMSO): δ 0.919 (t, CH2CH 3), 1.34 (s, C(CH3)3), 3.83 (m, CH2), 7.66 (d, aromatic H).
B. Deprotection of t-Boc-Glycine-Irinotecan
0.1 g t-Boc-Glycine-Irinotecan (0.137 mmoles) was dissolved in 7 mL of anhydrous DCM. To the solution was added 0.53 mL trifluoroacetic acid (6.85 mmoles). The solution was stirred at room temperature for 1 hour. The solvent was removed using rotary evaporation. The crude product was dissolved in 0.1 mL MeOH and then precipitated in 25 mL of ether. The suspension was stirred in an ice bath for 30 minutes. The product was collected by filtration and dried under vacuum. 1H NMR (DMSO): δ 0.92 (t, CH2CH 3), 1.29 (t, CH2CH 3), 5.55 (s, 2H), 7.25 (s, aromatic H).
C. Covalent Attachment of a Multi-Armed Activated Polymer to Glycine Irinotecan.
0.516 g Glycine-Irinotecan (0.976 mmoles), 3.904 g 4arm-PEG(20K)-CM (0.1952 mmoles), 0.0596 g 4-(dimethylamino)pyridine (DMAP, 0.488 mmoles), and 0.0658 g 2-hydroxybenzyltriazole (HOBT, 0.488 mmoles) were dissolved in 60 mL anhydrous methylene chloride. To the resulting solution was added 0.282 g 1,3-dicyclohexylcarbodiimide (DCC, 1.3664 mmoles). The reaction mixture was stirred overnight at room temperature. The mixture was filtered through a coarse frit and the solvent was removed using rotary evaporation. The syrup was precipitated in 200 mL of cold isopropanol over an ice bath. The solid was filtered and then dried under vacuum. Yield: 4.08 g. 1H NMR (DMSO): δ 0.909 (t, CH2CH 3), 1.28 (m, CH2CH 3), 3.5 (br m, PEG), 3.92 (s, CH2), 5.50 (s, 2H).
Example 2 ANTI-TUMOR ACTIVITY OF PENTAERYTHRITOLYL-4-ARM-(PEG -1-METHYLENE-2 OXO-VINYLAMINO ACETATE LINKED-IRINOTECAN)-20K, “4-ARM-PEG-GLY-IRINO-20K” IN A COLON CANCER MOUSE XENOGRAFT MODEL
Human HT29 colon tumor xenografts were subcutaneously implanted in athymic nude mice. After about two weeks of adequate tumor growth (100 to 250 mg), these animals were divided into different groups of ten mice each. One group was dosed with normal saline (control), a second group was dosed with 60 mg/kg of irinotecan, and the third group was dosed with 60 mg/kg of the 4-arm PEG-GLY-Irino-20K (dose calculated per irinotecan content). Doses were administered intraveneously, with one dose administered every 4 days for a total of 3 administered doses. The mice were observed daily and the tumors were measured with calipers twice a week. FIG.1 shows the effect of irinotecan and PEG-irinotecan treatment on HT29 colon tumors in athymic nude mice.
As can be seen from the results depicted in FIG. 1, mice treated with both irinotecan and 4-arm-PEG-GLY-Irino-20K exhibited a delay in tumor growth (anti-tumor activity) that was significantly improved when compared to the control. Moreover, the delay in tumor growth was significantly better for the 4-arm-PEG-GLY-Irino-20K group of mice when compared to the group of animals administered unconjugated irinotecan.
Example 3 SYNTHESIS OF PENTAERYTHRITOLYL-4-ARM-(PEG-1-METHYLENE-2 OXO-VINYLAMINO ACETATE LINKED-IRINOTECAN)-40K, “4-ARM-PEG-GLY-IRINO-40K”
4-arm-PEG-GLY-IRINO-40K was prepared in an identical fashion to that described for the 20K compound in Example 1, with the exception that in step C, the multi-armed activated PEG reagent employed was 4 arm-PEG(40K)-CM rather than the 20K material.
Example 4 SYNTHESIS OF PENTAERYTHRITOLYL-4-ARM-(PEG-1-METHYLENE-2 OXO-VINYLAMINO ACETATE LINKED-SN-38)-20K, “4-ARM-PEG-GLY-SN-38-20K”
4-arm PEG-GLY-SN-38-20K was prepared in a similar fashion to its irinotecan counterpart as described in Example 1, with the exception that the active agent employed was SN-38, an active metabolite of camptothecin, rather than irinotecan, where the phenolic-OH of SN-38 was protected with MEMCI (2-methoxyethoxymethyl chloride) during the chemical transformations, followed by deprotection with TEA to provide the desired multi-armed conjugate.
Example 5 SYNTHESIS OF PENTAERYTHRITOLYL-4-ARM-(PEG-1-METHYLENE-2 OXO-VINYLAMINO ACETATE LINKED-SN-38)-40K, “4-ARM-PEG-GLY-SN-38-40K”
4-arm PEG-GLY-SN-38-40K was prepared in a similar fashion to the 20K version described above, with the exception that the multi-armed activated PEG reagent employed was 4 arm-PEG(40K)-CM rather than the 20K material.
Example 8 SYNTHESIS OF PENTAERYTHRITOLYL-4-ARM-(PEG-2-{2-[2-1-HYDROXY-2-OXO-VINYLOXY)-ETHOXY]-ETHYLAMINO}-PROPEN-1-ONE LINKED-IRINOTECAN)-20K AND -40K
A. 2-(2-t-Boc-aminoethoxy)ethanol (1)
2-(2-Aminoethoxy)ethanol (10.5 g, 0.1 mol) and NaHCO3 (12.6 g, 0.15 mol) were added to 100 mL CH2Cl2 and 100 mL H2O. The solution was stirred at RT for 10 minutes, then di-tert-butyl dicarbonate (21.8 g, 0.1 mol) was added. The resulting solution was stirred at RT overnight, then extracted with CH2Cl2 (3×100 mL). The organic phases were combined and dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was subjected to silica gel column chromatography (CH2Cl2:CH3OH=50:1˜10:1) to afford 2-(2-t-Boc-aminoethoxy)ethanol (1) (16.0 g, 78 mmol, yield 78%)
B. 2-(2-t-Boc-aminoethoxy)ethoxycarbonyl-Irinotecan (2)
2-(2-t-Boc-aminoethoxy)ethanol (1) (12.3 g, 60 mmol) and 4-dimethylaminopyridine (DMAP) (14.6 g, 120 mmol) were dissolved in 200 ml anhydrous CH2Cl2. Triphosgene (5.91 g, 20 mmol) was added to the solution while stirring at room temperature. After 20 minutes, the solution was added to a solution of irinotecan (6.0 g, 10.2 mmol) and DMAP (12.2 g, 100 mmol) in anhydrous CH2Cl2 (200 mL). The reaction was stirred at RT for 2 hrs, then washed with HCI solution (pH=3, 2L) to remove DMAP. The organic phases were combined and dried over anhydrous sodium sulfate. The dried solution was evaporated under vacuum and subjected to silica gel column chromatography (CH2Cl2:CH3OH=40:1˜10:1) to afford 2-(2-t-Boc-aminoethoxy)ethoxycarbonyl-irinotecan (2) (4.9 g, 6.0 mmol, yield 59%).
C. 2-(2-aminoethoxy)ethoxycarbonyl-irinotecan TFA salt (3)
2-(2-t-Boc-aminoethoxy)ethoxycarbonyl-irinotecan (2) (4.7 g, 5.75 mmol) was dissolved in 60 mL CH2Cl2, and trifluoroacetic acid (TFA) (20 mL) was added at RT. The reaction solution was stirred for 2 hours. The solvents were removed under vacuum and the residue was added to ethyl ether and filtered to give a yellow solid as product 3 (4.3 g, yield 90%).
D. 4-arm-PEG20k-carbonate-inotecan (4)
4-arm-PEG20k-SCM (16.0 g) was dissolved in 200 mL CH2Cl2. 2-(2-aminoethoxy)ethoxycarbonyl-irinotecan TFA salt (3) (2.85 g, 3.44 mmol) was dissolved in 12 mL DMF and treated with 0.6 mL TEA, then added to a solution of 4-arm-PEG20k-SCM. The reaction was stirred at RT for 12 hrs then precipitated in Et2O to yield a solid product, which was dissolved in 500 mL IPA at 50° C. The solution was cooled to RT and the resulting precipitate collected by filtration to give 4-arm-PEG20k-glycine -irinotecan (4) (16.2 g, drug content 7.5% based on HPLC analysis). Yield: 60%.
E. 4-arm-PEG40k-carbonate-irinotecan (5)
4-arm-PEG40k-SCM (32.0 g) was dissolved in 400 mL CH2Cl2. 2-(2-aminoethoxy)ethoxycarbonyl-irinotecan TFA salt (3) (2.85 g, 3.44 mmol) was dissolved in 12 mL DMF and treated with 0.6 mL TEA, then added to the solution of 4-arm -PEG40k-SCM. The reaction was stirred at RT for 12 hrs and then precipitated in Et2O to get solid product, which was dissolved in 1000 mL isopropyl alcohol (IPA) at 50° C. The solution was cooled to RT and the precipitate collected by filtration to gave 4-arm-PEG40k-glycine-irinotecan (4) (g, drug content 3.7% based on HPLC analysis). Yield: 59%.
References
1: Iwase Y, Maitani Y. Dual functional octreotide-modified liposomal irinotecan leads to high therapeutic efficacy for medullary thyroid carcinoma xenografts. Cancer Sci. 2011 Oct 24. doi: 10.1111/j.1349-7006.2011.02128.x. [Epub ahead of print] PubMed PMID: 22017398.
2: Matsuzaki T, Takagi A, Furuta T, Ueno S, Kurita A, Nohara G, Kodaira H, Sawada S, Hashimoto S. Antitumor activity of IHL-305, a novel pegylated liposome containing irinotecan, in human xenograft models. Oncol Rep. 2012 Jan;27(1):189-97. doi: 10.3892/or.2011.1465. Epub 2011 Sep 20. PubMed PMID: 21935577.
3: Cobleigh MA. Other options in the treatment of advanced breast cancer. Semin Oncol. 2011 Jun;38 Suppl 2:S11-6. Review. PubMed PMID: 21600380.
4: Li C, Cui J, Wang C, Li Y, Zhang L, Xiu X, Li Y, Wei N, Zhang L, Wang P. Novel sulfobutyl ether cyclodextrin gradient leads to highly active liposomal irinotecan formulation. J Pharm Pharmacol. 2011 Jun;63(6):765-73. doi: 10.1111/j.2042-7158.2011.01272.x. Epub 2011 Apr 7. PubMed PMID: 21585373.
5: Iwase Y, Maitani Y. Octreotide-targeted liposomes loaded with CPT-11 enhanced cytotoxicity for the treatment of medullary thyroid carcinoma. Mol Pharm. 2011 Apr 4;8(2):330-7. Epub 2011 Jan 18. PubMed PMID: 21166471.
6: Xenidis N, Vardakis N, Varthalitis I, Giassas S, Kontopodis E, Ziras N, Gioulbasanis I, Samonis G, Kalbakis K, Georgoulias V. Α multicenter phase II study of pegylated liposomal doxorubicin in combination with irinotecan as second-line treatment of patients with refractory small-cell lung cancer. Cancer Chemother Pharmacol. 2011 Jul;68(1):63-8. Epub 2010 Sep 10. PubMed PMID: 20830475.
7: Pastorino F, Loi M, Sapra P, Becherini P, Cilli M, Emionite L, Ribatti D, Greenberger LM, Horak ID, Ponzoni M. Tumor regression and curability of preclinical neuroblastoma models by PEGylated SN38 (EZN-2208), a novel topoisomerase I inhibitor. Clin Cancer Res. 2010 Oct 1;16(19):4809-21. Epub 2010 Aug 11. PubMed PMID: 20702613.
8: Morgensztern D, Baggstrom MQ, Pillot G, Tan B, Fracasso P, Suresh R, Wildi J, Govindan R. A phase I study of pegylated liposomal doxorubicin and irinotecan in patients with solid tumors. Chemotherapy. 2009;55(6):441-5. Epub 2009 Dec 8. PubMed PMID: 19996589.
9: Meckley LM, Neumann PJ. Personalized medicine: factors influencing reimbursement. Health Policy. 2010 Feb;94(2):91-100. Epub 2009 Oct 7. PubMed PMID: 19815307.
10: Skak K, Søndergaard H, Frederiksen KS, Ehrnrooth E. In vivo antitumor efficacy of interleukin-21 in combination with chemotherapeutics. Cytokine. 2009 Dec;48(3):231-8. Epub 2009 Aug 25. PubMed PMID: 19709902.
11: Murphy CG, Seidman AD. Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer. 2009 Jun;9 Suppl 2:S58-65. Review. PubMed PMID: 19596644.
12: Fox ME, Guillaudeu S, Fréchet JM, Jerger K, Macaraeg N, Szoka FC. Synthesis and in vivo antitumor efficacy of PEGylated poly(l-lysine) dendrimer-camptothecin conjugates. Mol Pharm. 2009 Sep-Oct;6(5):1562-72. PubMed PMID: 19588994; PubMed Central PMCID: PMC2765109.
13: Atyabi F, Farkhondehfai A, Esmaeili F, Dinarvand R. Preparation of pegylated nano-liposomal formulation containing SN-38: In vitro characterization and in vivo biodistribution in mice. Acta Pharm. 2009 Jun;59(2):133-44. PubMed PMID: 19564139.
14: Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008 Aug 20;130(33):10876-7. Epub 2008 Jul 29. PubMed PMID: 18661992; PubMed Central PMCID: PMC2597374.
15: Scott LC, Yao JC, Benson AB 3rd, Thomas AL, Falk S, Mena RR, Picus J, Wright J, Mulcahy MF, Ajani JA, Evans TR. A phase II study of pegylated-camptothecin (pegamotecan) in the treatment of locally advanced and metastatic gastric and gastro-oesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2009 Jan;63(2):363-70. Epub 2008 Apr 9. PubMed PMID: 18398613.
16: Almubarak M, Newton M, Altaha R. Reinduction of bevacizumab in combination with pegylated liposomal Doxorubicin in a patient with recurrent glioblastoma multiforme who progressed on bevacizumab/irinotecan. J Oncol. 2008;2008:942618. Epub 2008 Sep 2. PubMed PMID: 19259336; PubMed Central PMCID: PMC2648641.
17: Krauze MT, Noble CO, Kawaguchi T, Drummond D, Kirpotin DB, Yamashita Y, Kullberg E, Forsayeth J, Park JW, Bankiewicz KS. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007 Oct;9(4):393-403. Epub 2007 Jul 24. PubMed PMID: 17652269; PubMed Central PMCID: PMC1994096.
18: Li YF, Fu S, Hu W, Liu JH, Finkel KW, Gershenson DM, Kavanagh JJ. Systemic anticancer therapy in gynecological cancer patients with renal dysfunction. Int J Gynecol Cancer. 2007 Jul-Aug;17(4):739-63. Epub 2007 Feb 16. Review. PubMed PMID: 17309673.
19: Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2006 Dec;28(10):719-40. PubMed PMID: 17235418.
20: Lokich J. Same-day pegfilgrastim and chemotherapy. Cancer Invest. 2005;23(7):573-6. PubMed PMID: 16305982.
21: Honig A, Rieger L, Sutterlin A, Kapp M, Dietl J, Sutterlin MW, Kämmerer U. Brain metastases in breast cancer--an in vitro study to evaluate new systemic chemotherapeutic options. Anticancer Res. 2005 May-Jun;25(3A):1531-7. PubMed PMID: 16033055.
Irinotecan
 |
 |
| Systematic (IUPAC) name |
|---|
(S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-
3,14-dioxo1H-pyrano[3’,4’:6,7]-indolizino[1,2-b]quinolin-
9-yl-[1,4’bipiperidine]-1’-carboxylate |
| Clinical data |
|---|
| Trade names | Camptosar |
|---|
| AHFS/Drugs.com | monograph |
|---|
| MedlinePlus | a608043 |
|---|
| Pregnancy cat. | D (Australia, United States) |
|---|
| Legal status | POM (UK), ℞-only (U.S.) |
|---|
| Routes | Intravenous |
|---|
| Pharmacokinetic data |
|---|
| Bioavailability | NA |
|---|
| Metabolism | Hepatic glucuronidation |
|---|
| Half-life | 6 to 12 hours |
|---|
| Excretion | Biliary and renal |
|---|
| Identifiers |
|---|
| CAS number | 100286-90-6  |
|---|
| ATC code | L01XX19 |
|---|
| PubChem | CID 60838 |
|---|
| DrugBank | DB00762 |
|---|
| ChemSpider | 54825  |
|---|
| UNII | 7673326042  |
|---|
| KEGG | D08086  |
|---|
| ChEMBL | CHEMBL481  |
|---|
| Chemical data |
|---|
| Formula | C33H38N4O6 e |
|---|
| Mol. mass | 586.678 g/mol (Irinotecan)
623.139 g/mol (Irinotecan hydrochloride)
677.185 g/mol (Irinotecan hydrochloride trihydrate)) |
|---|
..............
Irinotecan (Camptosar,
Pfizer; Campto,
Yakult Honsha) is a drug used for the treatment of cancer.
Mechanism
Irinotecan is activated by hydrolysis to
SN-38, an inhibitor of topoisomerase I. This is then inactivated by
glucuronidation by uridine diphosphate glucoronosyltransferase 1A1 (
UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription.
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
Side-effects
The most significant adverse effects of irinotecan are severe diarrhea and extreme suppression of the immune system.
Diarrhea
Irinotecan-associated diarrhea is severe and clinically significant, sometimes leading to severe dehydration requiring hospitalization or intensive care unit admission. This side-effect is managed with the aggressive use of antidiarrheals such as
loperamide or
Lomotil with the first loose bowel movement.
Immunosuppression
The immune system is adversely impacted by irinotecan. This is reflected in dramatically lowered
white blood cell counts in the blood, in particular the
neutrophils. The patient may experience a period of
neutropenia (a clinically significant decrease of neutrophils in the blood) while the bone marrow increases white cell production to compensate.
Pharmacogenomics
Irinotecan is converted by an enzyme into its active metabolite SN-38, which is in turn inactivated by the enzyme UGT1A1 by glucuronidation.
*28 variant patients
People with variants of the UGT1A1 called TA
7, also known as the "*28 variant", express fewer UGT1A1 enzymes in their liver and often suffer from
Gilbert's syndrome. During chemotherapy, they effectively receive a larger than expected dose because their bodies are not able to clear irinotecan as fast as others. In studies this corresponds to higher incidences of severe neutropenia and diarrhea.
[4]
In 2004, a clinical study was performed that both validated prospectively the association of the *28 variant with greater toxicity and the ability of genetic testing in predicting that toxicity before chemotherapy administration.
[4]
In 2005, the FDA made changes to the labeling of irinotecan to add
pharmacogenomics recommendations, such that irinotecan recipients with a
homozygous (both of the two gene copies) polymorphism in UGT1A1 gene, to be specific, the *28 variant, should be considered for reduced drug doses.
[5] Irinotecan is one of the first widely used chemotherapy agents that is dosed according to the recipient's genotype.
[6]
Research
Recently it was shown that antitumor activity of irinotecan against
glioblastoma can be enhanced by co-treatment with
statins.
[7] Similarly, it was shown that
berberine may enhance chemosensitivity to irinotecan in colon cancercells.
[8]
References
- Pommier, Y., Leo, E., Zhang, H., Marchand, C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 17: 421-433.
- New York Times Article http://www.nytimes.com/1996/06/18/science/new-cancer-drug-approved.html
- FDA Review Letter http://www.accessdata.fda.gov/drugsatfda_docs/appletter/1998/20571s8ltr.pdf
- Innocenti F, Undevia SD, Iyer L, et al. (April 2004). "Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan". J. Clin. Oncol. 22 (8): 1382–8. doi:10.1200/JCO.2004.07.173. PMID 15007088.
- Camptosar® irinotecan hydrochloride injection August 2010 http://labeling.pfizer.com/ShowLabeling.aspx?id=533
- O'Dwyer PJ, Catalano RB (October 2006). "Uridine diphosphate glucuronosyltransferase (UGT) 1A1 and irinotecan: practical pharmacogenomics arrives in cancer therapy". J. Clin. Oncol. 24 (28): 4534–8. doi:10.1200/JCO.2006.07.3031. PMID 17008691.
- Jiang PF (Jan 2014). "Novel anti-glioblastoma agents and therapeutic combinations identified from a collection of FDA approved drugs.". J Transl Med. 12. doi:10.1186/1479-5876-12-13. PMC 3898565. PMID 24433351.
- Yu M (Jan 2014). "Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB". J Mol Med Rep 9 (1): 249–54. doi:10.3892/mmr.2013.1762. PMID 24173769.
- DNA Topoisomerases and Cancer. Yves Pommier, Editor. Human Press. 2012
External links
AZERBAIJAN
 Caucasus Mountains in northern Azerbaijan.
Caucasus Mountains in northern Azerbaijan.
Justice for Khojaly
Bibi Heybat Mosque
///////