Buspirone
- Molecular FormulaC21H31N5O2
- Average mass385.503 Da
буспирон
بوسبيرون
丁螺酮
8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-8-aza-spiro[4.5]decane-7,9-dione
8-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione
- 8-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione
- Buspin
- Buspirone
- Spitomin
Buspirone
PAPER
https://pubs.rsc.org/en/content/articlelanding/2019/GC/C8GC03328E#!divAbstract
- Green Chemistry, 21(1), 59-63; 2019
Abstract
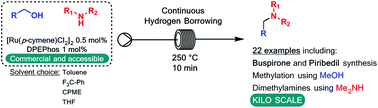
A continuous flow method for the direct conversion of alcohols to amines via a hydrogen borrowing approach is reported. The method utilises a low loading (0.5%) of a commercial catalyst system ([Ru(p-cymene)Cl2]2 and DPEPhos), reagent grade solvent and is selective for primary alcohols. Successful methylation of amines using methanol and the direct dimethylamination of alcohols using commercial dimethylamine solution are reported. The synthesis of two pharmaceutical agents Piribedil (5) and Buspirone (25) were accomplished in good yields employing these new methods.
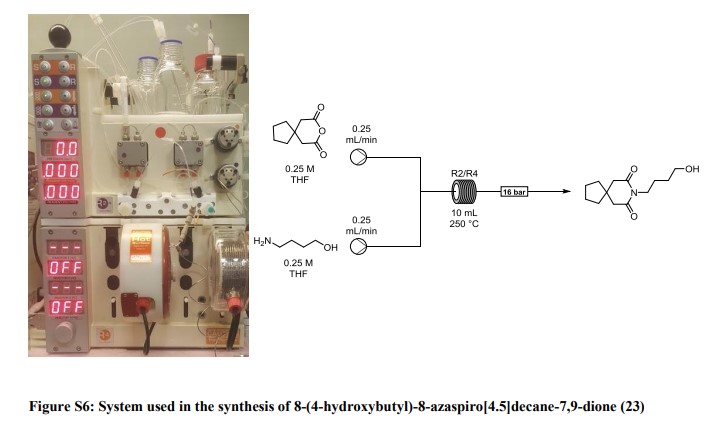
8-(4-hydroxybutyl)-8-azaspiro[4.5]decane-7,9-dione (23): A solution of 3,3-tetramethyleneglutaric anhydride (0.25 mol/L in THF) was combined in a tee piece with a solution of 4-amino-1-butanol (0.25 mol/L in THF) and reacted in a 20 mL reactor coil (stainless steel, 20 min residence time) heated at 250 °C. The output was concentrated in vacuo and the residue purified by column chromatography on silica gel to afford the product in 84% yield (Rf = 0.31, 63% DCM/AcOEt). 1H NMR (400 MHz, CDCl3) δ = 3.78 (t, J = 7.2 Hz, 2H), 3.65 (t, J = 6.0 Hz, 2H), 2.58 (s, 4H), 1.77 – 1.64 (m, 4H), 1.64 – 1.53 (m, 4H), 1.53 – 1.43 (m, 4H). 13C NMR (100 MHz, CDCl3) δ = 172.33, 62.28, 44.87, 39.47, 39.14, 37.54, 29.81, 24.35, 24.17. HRMS for [C13H22NO3] + calculated 240.1594 found 240.1605.

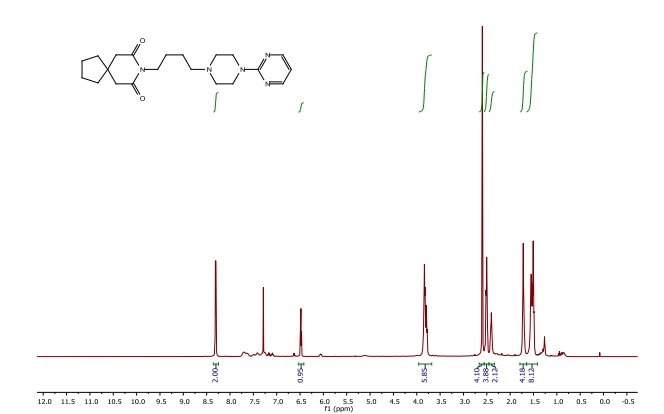
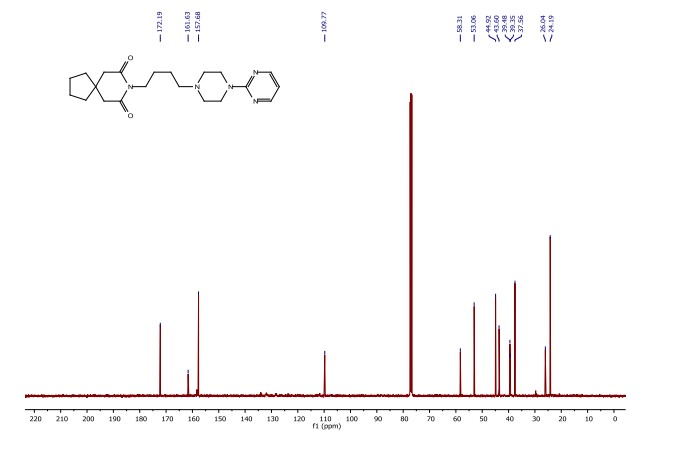
8-(4-(4-(pyrimidin-2-yl)piperazin-1-yl)butyl)-8-azaspiro[4.5]decane-7,9-dione (Buspirone, 25): The flow system was flushed with THF, the back-pressure regulator was set to 50 bar, and the coil reactor heated to 250 °C. Then a solution (10 mL overall volume) containing 1-(2-pyrimidyl)piperazine (2 mmol), 8-(4-hydroxybutyl)- 8-azaspiro[4.5]decane-7,9-dione (23) (2 mmol), dichloro(p-cymene)ruthenium(II) dimer (0.08 mmol) and bis[(2- diphenylphosphino)phenyl] ether (DPEPhos, 0.17 mmol) was pumped at 0.8 ml/min through a heated coil (8 mL, Phoenix reactor). The output solution obtained in steady state (monitored using the FlowUV) was concentrated in vacuo and purified by column chromatography on silica gel to afford the desired product in 76% yield (Rf = 0.29, 5% MeOH/DCM). 1H NMR (400 MHz, CDCl3) δ = 8.31 (d, J = 4.7 Hz, 2H), 6.48 (t, J = 4.7 Hz, 1H), 3.84 (t, J = 5.1 Hz, 4H), 3.79 (t, J = 6.8 Hz, 2H), 2.60 (s, 4H), 2.50 (t, J = 5.1 Hz, 4H), 2.40 (t, J = 6.8 Hz, 2H), 1.79 – 1.65 (m, 4H), 1.65 – 1.42 (m, 8H). 13C NMR (100 MHz, CDCl3) δ = 172.19, 161.63, 157.68, 109.77, 58.31, 53.06, 44.92, 43.60, 39.48, 39.35, 37.56, 26.04, 24.19, 24.19. HRMS for [C21H32N5O2] + calculated 386.2551 found 386.2570.


CAS Registry Number: 36505-84-7
CAS Name: 8-[4-[4-(2-Pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione
Molecular Formula: C21H31N5O2
Molecular Weight: 385.50
Percent Composition: C 65.43%, H 8.11%, N 18.17%, O 8.30%
Literature References: Non-benzodiazepine anxiolytic; 5-hydroxytryptamine (5-HT1) receptor agonist. Prepn: Y. H. Wu et al., J. Med. Chem. 15, 477 (1972); Y. H. Wu, J. W. Rayburn, DE 2057845 (1971 to Bristol-Myers); eidem, US 3717634 (1973 to Mead-Johnson). Pharmacology: L. E. Allen et al., Arzneim.-Forsch. 24, 917 (1974). Comparison with diazepam in treatment of anxiety: H. L. Goldberg, R. J. Finnerty, Am. J. Psychiatry 136, 1184 (1979); A. F. Jacobson et al., Pharmacotherapy 5, 290 (1985). Nonsynergistic effect with alcohol: T. Seppala et al., Clin. Pharmacol. Ther. 32, 201 (1982). Disposition and metabolism: S. Caccia et al., Xenobiotica 13, 147 (1983). Series of articles on chemistry, pharmacology, addictive potential, and clinical trials: J. Clin. Psychiatry 43, pp 1-116 (1982); on pharmacology, safety and clinical comparison with clorazepate: Am. J. Med. 80, Suppl. 3B, 1-51 (1986). Review of pharmacology and therapeutic efficacy: K. L. Goa, A. Ward, Drugs 32, 114-129 (1986). Review: M. W. Jann, Pharmacotherapy 8, 100-116 (1988); D. P. Taylor, FASEB J. 2, 2445-2452 (1988).
Derivative Type: Hydrochloride
CAS Registry Number: 33386-08-2
Trademarks: Ansial (Vita); Ansiced (Abello); Axoren (Glaxo Wellcome); Bespar (BMS); Buspar (BMS); Buspimen (Menarini); Buspinol (Zdravlje); Buspisal (Lesvi); Narol (Almirall)
Molecular Formula: C21H31N5O2.HCl
Molecular Weight: 421.96
Percent Composition: C 59.77%, H 7.64%, N 16.60%, O 7.58%, Cl 8.40%
Properties: Crystals from abs ethanol, mp 201.5-202.5°. LD50 i.p. in rats: 136 mg/kg (Allen).
Melting point: mp 201.5-202.5°
Toxicity data: LD50 i.p. in rats: 136 mg/kg (Allen)
Therap-Cat: Anxiolytic.
Keywords: Anxiolytic; Arylpiperazines; Serotonin Receptor Agonist.