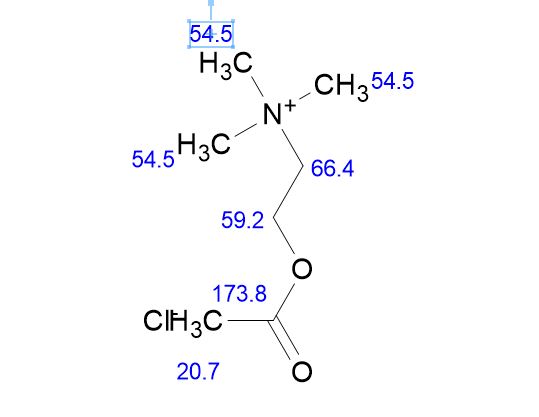
Acetylcholine Chloride
2-acetyloxyethyl(trimethyl)azanium;chloride
60-31-1
| Molecular Formula: | C7H16ClNO2 |
|---|---|
| Molecular Weight: | 181.66 g/mol |
Acetylcholine chloride is obtained as white or off-white hygroscopic crystals, or as a crystalline powder. The salt is odorless, or nearly odorless, and is a very deliquescent powder. Acetylcholine bromide is obtained as deliquescent crystals, or as a white crystalline powder. The substance is hydrolyzed by hot water and alkali
Acetylcholine is an organic chemical that functions in the brain and body of many types of animals, including humans, as a neurotransmitter—a chemical released by nerve cells to send signals to other cells. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that interfere with acetylcholine activity are called anticholinergics.
Acetylcholine is the neurotransmitter used at the neuromuscular junction—in other words, it is the chemical that motor neurons of the nervous system release in order to activate muscles. This property means that drugs that affect cholinergic systems can have very dangerous effects ranging from paralysis to convulsions. Acetylcholine is also used as a neurotransmitter in the autonomic nervous system, both as an internal transmitter for the sympathetic nervous system and as the final product released by the parasympathetic nervous system.
Inside the brain, acetylcholine functions as a neuromodulator—a chemical that alters the way other brain structures process information rather than a chemical used to transmit information from point to point. The brain contains a number of cholinergic areas, each with distinct functions. They play an important role in arousal, attention, and motivation.
Partly because of its muscle-activating function, but also because of its functions in the autonomic nervous system and brain, a large number of important drugs exert their effects by altering cholinergic transmission. Numerous venoms and toxins produced by plants, animals, and bacteria, as well as chemical nerve agents such as Sarin, cause harm by inactivating or hyperactivating muscles via their influences on the neuromuscular junction. Drugs that act on muscarinic acetylcholine receptors, such as atropine, can be poisonous in large quantities, but in smaller doses they are commonly used to treat certain heart conditions and eye problems. Scopolamine, which acts mainly on muscarinic receptors in the brain, can cause delirium and amnesia. The addictive qualities of nicotine derive from its effects on nicotinic acetylcholine receptors in the brain.
Chemistry
Acetylcholine is a choline molecule that has been acetylated at the oxygen atom. Because of the presence of a highly polar, charged ammonium group, acetylcholine does not penetrate lipid membranes. Because of this, when the drug is introduced externally, it remains in the extracellular space and does not pass through the blood–brain barrier. A synonym of this drug is miochol.
History
Acetylcholine (ACh) was first identified in 1915 by Henry Hallett Dale for its actions on heart tissue. It was confirmed as a neurotransmitter by Otto Loewi, who initially gave it the name Vagusstoff because it was released from the vagus nerve. Both received the 1936 Nobel Prize in Physiology or Medicine for their work. Acetylcholine was also the first neurotransmitter to be identified.
CLIP
Laboratory Synthesis Of Acetylcholine chloride
Acetylcholine chloride Chemical Name: 2- (acetyl oxy)- N ,N ,N- tri methyl ethan aminium chloride
Acetylcholine chloride Use: parasympathomimetic, miotic, vasodilator (peripheral)
Acetylcholine chloride MW: 181.66
Acetylcholine chloride MF: C7H16ClNO2
Acetylcholine chloride LD50: 10 mg/kg (M, i.v.); 3 g/kg (M, p.o.);
22 mg/kg (R, i.v.); 2500 mg/kg (R, p.o.)
Reference(s):
Acetylcholine chloride Use: parasympathomimetic, miotic, vasodilator (peripheral)
Acetylcholine chloride MW: 181.66
Acetylcholine chloride MF: C7H16ClNO2
Acetylcholine chloride LD50: 10 mg/kg (M, i.v.); 3 g/kg (M, p.o.);
22 mg/kg (R, i.v.); 2500 mg/kg (R, p.o.)
Reference(s):
- Baeyer, A. v.: Justus Liebigs Ann. Chem. (JLACBF) 142, 235 (1867).
- Nothnagel: Arch. Pharm. (Weinheim, Ger.) (ARPMAS) 232, 265 (1894).
- Fourneau, E.; Page, H.J.: Bull. Soc. Chim. Fr. (BSCFAS) [4] 15, 544 (1914).
- DE 801 210 (BASF; appl. 1948).
- US 1 957 443 (Merck & Co.; 1934; appl. 1931).
- US 2 012 268 (Merck & Co.; 1935; appl. 1931).
- US 2 013 536 (Merck & Co.; 1935; appl. 1931).
| Acetylcholine | |
|---|---|
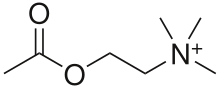 | |
| IUPAC name | 2-Acetoxy-N,N,N-trimethylethanaminium |
| Abbreviation | ACh |
| Sources | motor neurons, parasympathetic nervous system, brain |
| Targets | skeletal muscles, brain, many other organs |
| Receptors | nicotinic, muscarinic |
| Agonists | nicotine, muscarine, cholinesterase inhibitors |
| Antagonists | tubocurarine, atropine |
| Precursor | choline, acetyl-CoA |
| Synthesizing enzyme | choline acetyltransferase |
| Metabolizing enzyme | acetylcholinesterase |
| Database links | |
| CAS Number | 51-84-3 |
| PubChem | CID: 187 |
| IUPHAR/BPS | 294 |
| DrugBank | EXPT00412 |
| ChemSpider | 182 |
| KEGG | C01996 |


1H NMR PREDICT
13 C NMR PREDICT
/////////CC(=O)OCC[N+](C)(C)C.[Cl-]