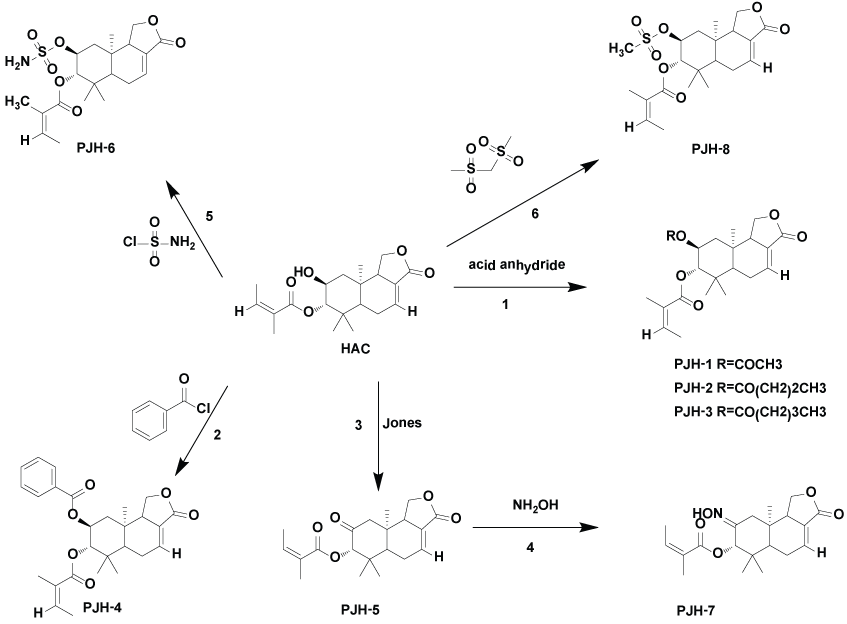
A sesequiterpenoid, 2α-hydroxyl-3β-angeloylcinnamolide (HAC) was isolated from the Chinese medicinal herb Polygonum jucundum Lindex. (Polygonaceae) with anti-inflammatory activities in vivo. In the present study, we investigated the anti-inflammation effects of HAC on lipopolysaacharide (LPS)-induced murine RAW264.7 cells. As the results, we found that HAC dose-dependently decreased NO over-production with IC50 value of 17.68 μM but showed very weak inhibition on TNF-α release with IC50 value of 98.66 μM. Meanwhile, eight novel derivatives modified at C-2 position of HAC were synthesized to further explore the structure-activity relationships (SARs) of HAC on antiinflammation effects. Compound PJH-1, an acetyl easer of HAC, showed better inhibition on over-production of NO and TNF-α (IC50, 7.31 and 3.38 μM, respectively). Furthermore, we demonstrated that HAC and PJH-1 attenuates the mitogen-activated protein kinases (MAPK) signaling pathways through blocking the phosphorylation of ERK, p38, JNK/MAPK. We also found that the structure of PJH-1 are more stable than that of HAC in cell medium, these finding are useful to develop in vitro molecular mechanism research of HAC. In a conclusion, our studies enhance the understanding of anti-inflammation activities of HAC and lead to the discovery of novel derivatives as potential antiinflammation agents.

Inflammation is the first response of a tissue to injury, it can be classed as both acute and chronic inflammations. Chronic inflammation is a persistent one, which cause progressive damage to the body [1]. Macrophages play a key role in the specific and non-specific immune responses during the inflammation process, after macrophages are activated by LPS, large amounts of the cytokines and inflammatory mediators will be released [2-4]. Among the many pro-inflammatory mediators, NO is a key one in inflammation reactors [5] which is a free radical produced from L-argine by nitric oxide synthases (NOS) and is known to regulate various physiological functions in many tissues [6], however, excessive NO has been implicated in various pathological processes. The inhibition of NO overproduction has been suggested to be an important therapeutic approach for treatment of inflammation [7,8]. Expression of the iNOS in macrophages is regulated mainly at the induction of transcription factors through mitogen-activated protein kinases (MAPKs).
The aerial parts of Polygonum jucundum Lindex. (Polygonaceae) is used as traditional Chinese herbs for inhibiting inflammation, lowering serum cholesterol levels, and treating rheumatism [9-11]. In our previous study, a drimane-type sesquiterpenoid, 2α- hydroxyl - 3β – angeloyl -cinnamolide (HAC) from P. jucundum, was identified with anti-inflammatory effects by oral administration effects by oral administration at dose of 50-200mg/kg in mouse, and a sensitive and rapid LC-MS method was developed to study its pharmacokinetics and distribution in rats [12-14]. Up to now, some natural sesquiterpenoids were shown to possess significant inhibitions on pro-inflammation mediator production [15-17]. The drimane-type sesquiterpenoids has been identified with anti-inflammatory properties [18]. Therefore, in this study, we investigated the effects of HAC on the release of LPS-induced pro-inflammatory mediators and explored the molecular mechanism in terms of inflammatory signaling pathways. Meanwhile, eight novel derivatives modified at carbon-2 position of HAC were synthesized (Scheme 1) to further explore structure-activity relationships (SARs) of HAC on LPS-activated RAW264.7 cells. These studies also lead to a better understanding of the structure-activity relationship for the sesquiterpene lactones family and the discovery of novel derivatives as potential anti-inflammation agents.
2α- acetoxy- 3β- angeloylcinnamolide (PJH-1 C22H30O6):
To a solution of HAC (200 mg, 0.574 mmol) in di-chloromethane (20 mL) was added DMAP (60 mg) and acetic anhydride (0.54 mL, 1.7 mmol). The reaction mixture was stirred at room temperature for 3 h (TLC monitoring). The crude product was chromatographed on a silica gel column using the elution (petroleum ether: acetyl acetate=1:1) to afford PJH-1 (83% yield) as a white needle crystal. Yield: 83%; white needle crystal. [α]20D=-0.73° (c 0.3, CH2Cl2). 1H-NMR (CDCl3, 500 MHz): δ2.02 (1H, dd, J=4.5, 12.5 Hz, H-1β), 1.46 (1H, t, J=12.1, 12.1 Hz, H-1α), 5.16 (1H, m, H-2), 4.94 (1H, d, J=9.0 Hz, H-3), 1.62 (1H, q, J=5.5 Hz, H-5), 2.47 (1H, m, H-6α), 2.25 (1H, m, H-6β), 6.90 (1H, m, H-7), 2.90 (1H, m, H-9), 4.39 (1H, t, J=9.0 Hz, H-11α), 4.05 (1H, t, J=9.0 Hz, H-11β), 1.26 (3H, s, H-13), 1.08 (3H, s, H-14), 0.97 (3H,s, H-15), angelica acyl: δ6.09 (1H, ddd, J=1.5, 1.5, 14.5 Hz, H-3'), 1.98 (3H, dd, J=1.5, 7.5 Hz, 3'-CH3), 1.89 (3H, t, J=1.5 Hz, 2'-CH3), Acetyl: 1.96 (3H, s, H-2'). 13C-NMR (CDCl3, 75 MHz): δ42.6 (C-1), 66.7 (C- 2), 79.2(C-3), 39.2(C-4), 48.9(C-5), 24.7(C-6), 135.7(C-7), 126.9(C-8), 50.6(C-9), 35.1(C-10), 68.6(C-11), 167.4(C-12), 17.1(C-13), 28.1(C- 14), 14.3(C-15), angelica acyl: 167.4(C-1'), 127.7(C-2'), 138.4(C-3'), 20.9(2'-CH3), 15.7(3'-CH3), acetyl: 167.4(C-1''), 20.5(2'-CH3).
Anti-inflammatory Properties of an Active Sesquiterpene Lactone and its Structure-Activity Relationship
Yang Hu1, Fei Zhang2, Chaofeng Zhang1* and Mian Zhang1
1State Key Laboratory of Natural Medicines, Research Department of Pharmacognosy, China Pharmaceutical University, Longmian Road 639, Nanjing 211198, PR China
2Jiangsu Simcere Pharmaceutical Group Ltd., Xuanwu Avenue No. 699-18, Nanjing 210042, PR China
Chaofeng Zhang
State Key Laboratory of Natural Medicines
Research Department of Pharmacognosy
China Pharmaceutical University
Longmian Road 639, Nanjing 211198
PR China
Tel/Fax: (86)-25-86185140

Citation: Hu Y, Zhang F, Zhang C, Zhang M (2015) Anti-inflammatory Properties of an Active Sesquiterpene Lactone and its Structure-Activity Relationship. Med chem 5:354-360. doi: 10.4172/2161-0444.1000286

Mian Zhang - China Pharmaceutical University.




//////