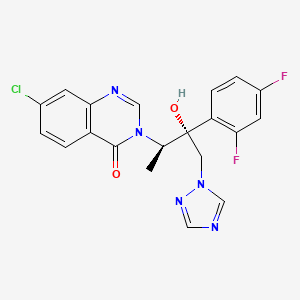
Albaconazole
Also known as: UNII-YDW24Y8IAB; UR-9825; 187949-02-6; UR 9825, W-0027
Molecular Formula: C20H16ClF2N5O2 Molecular Weight: 431.823146
(1R,2R)-7-chloro-3-[2-(2,4-difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]quinazolin-4(3H)-one
7-chloro-3-[(2R,3R)-3-(2,4-difluorophenyl)-3-hydroxy-4-(1,2,4-triazol-1-yl)butan-2-yl]quinazolin-4-one
J. Med. Chem., 1998, 41 (11), pp 1869–1882
DOI: 10.1021/jm9707277
A series of azole antifungal agents featuring a quinazolinone nucleus have been subjected to studies of structure−activity relationships. In general, these compounds displayed higher in vitro activities against filamentous fungi and shorter half-lives than the structures described in our preceding paper. The most potent products in vitro carried a halogen (or an isostere) at the 7-position of the quinazolinone ring. Using a murine model of systemic candidosis, oral activity was found to be dependent on hydrophobicity, which, in turn, modulated the compound’s half-life. The 7-Cl derivative, (1R,2R)-7-chloro-3-[2-(2,4-difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]quinazolin-4(3H)-one (20, UR-9825), was selected for further testing due to its high in vitro activity, low toxicity, good pharmacokinetic profile, and ease of obtention. Compound 20 is the (1R,2R) isomer of four possible stereoisomers. The other three isomers were also prepared and tested. The enantiomer (1S,2S) and the (1R,2S) epimer were inactive, whereas the (1S,2R) epimer retained some activity. In vitro 20 was superior to fluconazole, itraconazole, SCH-42427, and TAK-187 and roughly similar to voriconazole and ER-30346. In vivo, 20 was only moderately active in a mouse model of systemic candidosis when administration was limited to the first day. This was attributed to its short half-life in that species (t1/2 = 1 h po). Protection levels comparable to or higher than those of fluconazole, however, were observed in systemic candidosis models in rat and rabbit, where the half-life of the compound was found to be 6 and 9 h, respectively. Finally, 20 showed excellent protection levels in an immunocompromised rat model of disseminated aspergillosis. The compound showed low toxicity signs when administered to rats at 250 mg/kg qd or at 100 mg/kg bid during 28 days.
(1R,2R)-7-Chloro-3-[2-(2,4-difluorophenyl)-2-hydroxy-1-methyl-3-(1H-1,2,4-triazol-1-yl)propyl]quinazolin-4(3H)-one (20, UR-9825). Precipitated from EtOH/H2O (66% yield from amine 11): white amorphous solid;
mp 93−110 °C (wide range);
IR (KBr) ν 1675, 1601, 1554, 1498 cm-1;
1H NMR (300 MHz, CDCl3) 8.58 (s, 1H, N CH-N), 8.26 (d, J = 8.6, 1H, arom), 8.11 (d, J = 5.7, trace rotamer), 7.76 (s, 2H, triazol),
CH-N), 8.26 (d, J = 8.6, 1H, arom), 8.11 (d, J = 5.7, trace rotamer), 7.76 (s, 2H, triazol),
7.74 (d, J = 5.3, 1H, arom), 7.5 (m, 2H, arom), 7.10 (s, trace rotamer), 6.9−6.7 (m, 2H, arom),
5.91 (dq, Jd = 2, Jq = 7, 1H, MeCH), 5.54 (d, J = 2, 1H, OH),
5.15 (d, J = 14.2 1H, CH(H)), 4.9−4.7 (m, trace rotamer), 4.30 (d, trace rotamer), 3.99 (d, J = 14.2, 1H, CH(H)),
1.46 (d, J = 6.9, trace rotamer), 1.29 (d, J = 7, 3H, CHMe);
GC−MS 224 (Tr-CH2COHAr, C10H8F2N3O), 208 (group N-ethylheterocycle, C10H9ClN2O);
[α]D −8.0° (c 1, CHCl3).
Chiral HPLC (column, CicloBond SN 1; eluent, MeOH: Et3NHOAc in H2O at pH7 1:1; retention times: (S,S) (74) tR 12.6 min; (R,R) (20) tR 13.7 min). Area ratio: 0.01:99.99.
Anal. (C20H16ClF2N5O2) C, H, N.
synthesis at