1H NMR
13C NMR
Org. Chem. Front., 2017, Advance Article
DOI: 10.1039/C7QO00021A, Research Article
DOI: 10.1039/C7QO00021A, Research Article
Junliang Wang, Jianneng Li, Xianwang Shen, Cong Dong, Jun Lin, Kun Wei
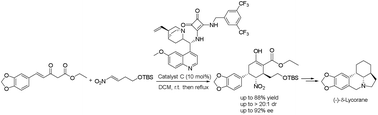
A first asymmetric synthesis of (-)-δ-lycorane by using a chiral bifunctional squaramide-catalysed cascade reaction is reported.
A first asymmetric synthesis of (-)-δ-lycorane by using a chiral bifunctional squaramide-catalysed cascade reaction is reported.
*Corresponding authors
aSchool of Chemical Science and Technology, Yunnan University, P. R. China
bSchool of Chemical Science and Technology, Yunnan University, Key Laboratory of Medicinal Chemistry for Natural Resource (Ministry of Education), Kunming, P. R. China
E-mail: weikun@ynu.edu.cn, linjun@ynu.edu.cn
Fax: +86871 65031633
Tel: +86871 65031633
E-mail: weikun@ynu.edu.cn, linjun@ynu.edu.cn
Fax: +86871 65031633
Tel: +86871 65031633
Org. Chem. Front., 2017, Advance Article
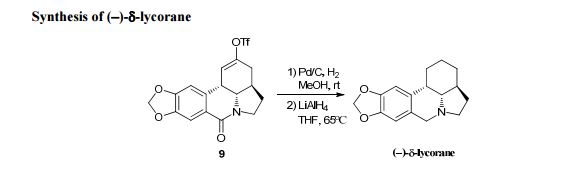
A first asymmetric synthesis of the lycorine-type Amaryllidaceae alkaloid (−)-δ-lycorane by using a chiral bifunctional squaramide-catalysed cascade reaction as a powerful tool to construct the skeleton of the alkaloid on the basis of unsaturated β-ketoester and nitroalkene is reported. The sequence afforded a highly functionalized intermediate with three stereogenic centres with high diastereoselectivity (>20 :
: 1 dr) and high enantioselectivity (92% ee) in one step, which was converted into (−)-δ-lycorane in eight steps.
1 dr) and high enantioselectivity (92% ee) in one step, which was converted into (−)-δ-lycorane in eight steps.
 :
: 1 dr) and high enantioselectivity (92% ee) in one step, which was converted into (−)-δ-lycorane in eight steps.
1 dr) and high enantioselectivity (92% ee) in one step, which was converted into (−)-δ-lycorane in eight steps.
The residue was purified by flash silica 8 Please do not adjust margins Please do not adjust margins Please do not adjust margins gel chromatography (DCM/MeOH = 10/1) to give (−)-δ-lycorane (9 mg, 72%) as a white solid.
m.p.: 125-126 °C; [α] = -51.9, (c = 0.1, CHCl3);
IR (thin film, ν cm -1): 3551, 3477, 3414 , 1637, 1617, 619, 473;
1H-NMR (500 MHz, CDCl3), δ (ppm): 6.79 (s, 1H), 6.68 (s, 1H), 5.93 (s, 2H), 4.29 (d, J = 14.5 Hz, 1H), 3.95 (dd, J = 11.0, 8.5 Hz, 1H), 3.68 (d, J = 14.5 Hz, 1H), 3.52 (dd, J = 11.5, 4.0 Hz, 1H), 3.25 (s, 1H), 2.50 (m, 1H), 2.41 (d, J = 2.0 Hz, 1H), 1.86 (m, 1H), 1.77 (m, 2H), 1.66 (d, J = 14.5 Hz, 1H), 1.59 (m, 2H), 1.37 (m, 2H);
13C-NMR (125 MHz, CDCl3) δ (ppm): 149.0, 146.5, 131.3, 120.5, 109.5, 106.9, 101.5, 66.3, 55.1, 50.7, 39.7, 34.5, 29.6, 29.0, 24.8, 20.4;
HRMS (ESI): m/z calcd for C16H20NO2 + [M + H]+ : 258.1489, found: 258.1488.

////(−)-δ-lycorane