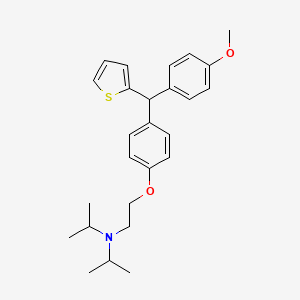
CDRI 830
CDRI S006-830
S 006-830
CAS 1550975-42-2
N-[2-[4-[(4-methoxyphenyl)-thiophen-2-ylmethyl]phenoxy]ethyl]-N-propan-2-ylpropan-2-amine
| Molecular Formula: | C26H33NO2S |
|---|---|
| Molecular Weight: | 423.61072 g/mol |

CDRI-830 of thiophene containing trisubstituted methane (TRSM) class was identified as an anti-tubercular lead with MIC value of 1.33 mg/L against Mycobacterium tuberculosis H37Rv strain, non-toxicity against Vero C-1008 cell line (selectivity index >10), ex vivo efficacy (in mouse and human macrophages) equivalent to first line TB drugs, lung CFU count (2.2×107) comparable to pyrazinamide (1.9×107) and ethambutol (1.27×107). CDRI-830 has exhibited potent bactericidal activity against single and multi-drug resistant clinical isolates of M. tuberculosis. Furthermore, CDRI-830 has demonstrated good pharmacokinetic properties with fast intestinal absorption, peak plasma concentration one hour post oral dose, optimum elimination half-life (9-13 h), plasma protein binding (~60%), favorable bioavailability (45-50%) and mean residence time (18-20 h).

CDRI S006-830 is a potent triethylamine containing thiophene antitubercular compound of the Central Drug Research Institute, India. The present study aimed to conduct comprehensive metabolic investigations of CDRI S006-830 to corroborate its preclinical investigations. Preliminary metabolic investigations were performed to assess the metabolic stability, enzyme kinetics, reaction phenotyping, and metabolite identification of CDRI S006-830 in rat, rabbit, dog, and human liver microsomes using liquid chromatography with mass spectrometry. The observed in vitro t1/2 and Clint values were 9.9 ± 1.29, 4.5 ± 0.52, 4.5 ± 0.86, 17 ± 5.21 min and 69.60 ± 8.37, 152.0 ± 17.26, 152.34 ± 27.63, 33.62 ± 21.04 μL/min/mg in rat, rabbit, dog and human liver microsomes respectively. These observations suggested that CDRI S006-830 rapidly metabolized in the presence of NADPH in liver microsomes of rat, rabbit and dog while moderately metabolized in human liver microsomes. It was observed that CDRI S006-830 exhibited monophasic Michaelis–Menten kinetics. The metabolism of CDRI S006-830 was primarily mediated by CYP3A4 and was deduced by CYP reaction phenotyping with known potent inhibitors. CYP3A4 involvement was also confirmed by cDNA-expressed recombinant human isozyme activity with different CYPs. Four major phase-I metabolites of S006-830, (M-1 to M-4) were detected in rat, rabbit, dog (except M4) and human liver microsomes........http://onlinelibrary.wiley.com/doi/10.1002/dta.1802/abstract?systemMessage=Wiley+Online+Library+will+be+unavailable+on+Saturday+14th+May+11%3A00-14%3A00+BST+%2F+06%3A00-09%3A00+EDT+%2F+18%3A00-21%3A00+SGT+for+essential+maintenance.Apologies+for+the+inconvenience.
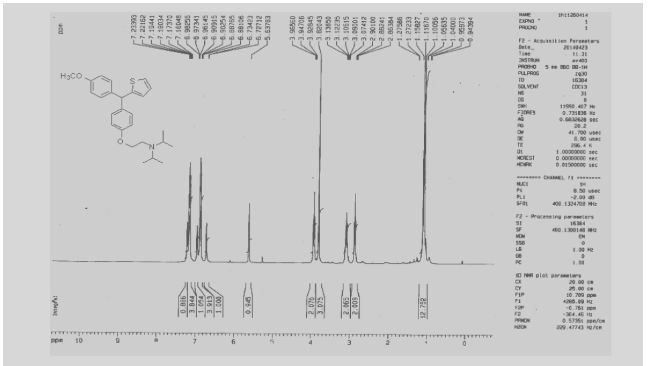
13C NMR
SYNTHESIS
Gautam Panda
| Associate Professor AcSIR ( Academy of Scientific and Innovative Research, New Delhi, India) Principal Scientist and Group Leader Medicinal and Process Chemistry Division CSIR-CDRI ( Central Drug Research Institute ) Sector-10, Jankipuram Extension, Sitapur Road, Lucknow-226031 Phone (Office) : 0522-2772450, 2772550, Ext. 4661, 4662 Phone (Res.) : 0522-2746635 Fax : 0522-2771941 Email : gautam.panda@gmail.com, gautam_panda@cdri.res.inWebpage: http://www.cdriindia.org/gautampanda.htm |
PATENT
Indian Pat. Appl. (2012), IN 2010DE00685
Abstract:
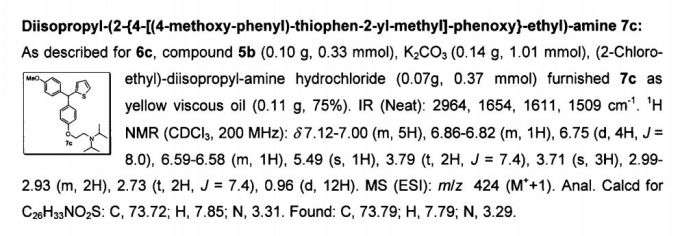
The invention relates to Thiophene containing Trisubstituted Methanes (TRSMs) and a process for the preparation thereof. The invention particularly relates to a process for the preparation of substituted secondary and tertiary amino alkoxy diary! thiophenyl methanes and their use as potential antimycobacterial agents. Novel diaryl thiophenyl methanes of formula I have been prepared. The present invention provides novel diaryl thiophenyl methanes substituted with a secondary or tertiary amino alkoxy group and a process for the preparation of the said compounds of general formula I comprising general formula la and lb useful in antimycobacterial activity wherein R1 is selected from an aryl group or thophene moiety wherein the aryl group is selected from a group consisting of substituted phenyl groups such as methoxy phenyl, thiomethoxy phenyl, phenyl, p-chlorophenyl, p-fluorophenyl; R2 is selected from a group consisting of aminoalkoxyl, alkyl/dialkyl aminoalkoxy, cyclic alkyl aminoalkoxy. R3 is selected from a group consisting of H, lower alkyl, lower alkoxy group such as methyl, ethyl, n-propyl, isopropyl, n-butyl, secondary butyl, tertiary butyl, n-amyl, n-hexyl, 2-ethyl butyl; R4 is selected from a group consisting of H,OH,methyl.
The invention relates to Thiophene containing Trisubstituted Methanes (TRSMs) and a process for the preparation thereof. The invention particularly relates to a process for the preparation of substituted secondary and tertiary amino alkoxy diary! thiophenyl methanes and their use as potential antimycobacterial agents. Novel diaryl thiophenyl methanes of formula I have been prepared. The present invention provides novel diaryl thiophenyl methanes substituted with a secondary or tertiary amino alkoxy group and a process for the preparation of the said compounds of general formula I comprising general formula la and lb useful in antimycobacterial activity wherein R1 is selected from an aryl group or thophene moiety wherein the aryl group is selected from a group consisting of substituted phenyl groups such as methoxy phenyl, thiomethoxy phenyl, phenyl, p-chlorophenyl, p-fluorophenyl; R2 is selected from a group consisting of aminoalkoxyl, alkyl/dialkyl aminoalkoxy, cyclic alkyl aminoalkoxy. R3 is selected from a group consisting of H, lower alkyl, lower alkoxy group such as methyl, ethyl, n-propyl, isopropyl, n-butyl, secondary butyl, tertiary butyl, n-amyl, n-hexyl, 2-ethyl butyl; R4 is selected from a group consisting of H,OH,methyl.
The invention relates to thiophene containing Trisubstituted Methanes (TRSMs) and a process for the preparation thereof. The invention particularly relates to a process for the preparation of substituted secondary and tertiary amino alkoxy diaryl thiophenyl methanes and their use as potential antimycobacterial agents. Novel diaryl thiophenyl methanes of formula I have been prepared.
The present invention provides novel diaryl thiophenyl methanes substituted with a secondary or tertiary amino alkoxy group and a process for the preparation of the said compounds of general formula I comprising formula la and lb useful in antimycobacterial activity wherein R1 is selected from an aryl group or thophene moiety wherein the aryl group is selected from a group consisting of substituted phenyl groups such as methoxy phenyl, thiomethoxy phenyl, phenyl, p-chlorophenyl, p-fluorophenyl; R2 is selected from a group consisting of aminoalkoxyl, alkyl/dialkyl aminoalkoxy, cyclic alkyl aminoalkoxy. R3 is selected from a group consisting of H, lower alkyl, lower alkoxy group such as methyl, ethyl, n-propyl, isopropyl, n-butyl, secondary butyl, tertiary butyl, n-amyl, n-hexyl, 2-ethyl butyl ; R4 is selected from a group consisting of H,OH,methyl etc.
(Formula Removed)
Background of the Invention
Tuberculosis is a growing international health concern; it is the leading infectious cause of death in the world today (Dolin, P.J. et al Bull. WHO 1994, 72, 213; Daffe, M. et al Adv. Microb. Physiol 1998, 39, 131). It is estimated that worldwide 100 million people are infected annually.
Approximately ten million develop the disease, with five million of these progressing to the infectious stage and ultimately three million dying. Even though improved methods of prevention, detection, diagnosis and modern treatment have greatly reduced the number of people getting infected and dying from it, the emergence of multi-drug-resistant (MDR) strains and the global human immunodeficiency virus (HIV) augments the risk of developing TB many fold. Resistance has been described for all first-line drugs (isoniazid, rifampin, pyrazinamide, ethambutol and streptomycin) and for several second-line and newer drugs (ethionamide, fluoroquinolones, macrolides, nitroimidazopyrans). Because MDR strains are the result of cumulative mutations, growth of Mycobacterium tuberculosis (MT) can successfully be controlled in the host by concomitant treatment with more than one drug. This has resulted in the development of new agents (Panda, G. et al Indian Journal of Chemistry, 2009, 48B, 1121-1127; Parai, M. K. et al Bioorganic & Medicinal Chemistry Letters, 2008, 18, 289-292) for the preparation of Disseminated Mycobacterium avium complex (DMAC) infection as well as combinations of both new and standard agents for its treatment. The search for more effective agents against Mycobacterium tuberculosis (MT) and Mycobacterium avium complex (MAC) is ongoing in an attempt to enhance survival and reduce morbidity, as proven by the high number of publications (Jing-Ping Lu et al J. Med. Chem., 2010, 53, 3, 1329-1337; Liqiang Chen et al J. Med. Chem., 2010, 53 (12), 4768-4778; Jiyoung A et al J. Med. Chem., 2009, 52 (17), 5485-5495; Maria-Teresa Gutierrez-Lugo et al J. Med. Chem., 2008, 51 (9), 2606-2612; Li Liu et al J. Med. Chem., 2010, 53 (7), 2882-2891 and references cited therein) and patents of new antituberculous drugs recently published. Preclinical data, such as in vitro measures of drug activity and pharmacokinetics, are used in the design of new treatment regimens. Assessment of pharmacodynamic activity from standard in vitro minimum inhibitory concentrations (MICs) alone is insufficient to predict in vivo potency. Achievable serum and tissue concentrations as well as pharmacokinetic characteristics must be considered.
Because of this, there is an urgent need for anti-TB drugs with improved properties such as enhanced activity against MDR strains, reduced toxicity, shortened duration of therapy, rapid mycobactericidal mechanism of action and the ability to penetrate host cells and exert anti-mycobacterial effects in the intracellular environment.
Following is the description of thiophene containing trisubstituted methanes having antimycobacterial activity.
The present invention provides novel diaryl thiophenyl methanes substituted with a secondary or tertiary amino alkoxy group and a process for the preparation of the said compounds of general formula I comprising formula la and lb useful in antimycobacterial activity wherein R1 is selected from an aryl group or thophene moiety wherein the aryl group is selected from a group consisting of substituted phenyl groups such as methoxy phenyl, thiomethoxy phenyl, phenyl, p-chlorophenyl, p-fluorophenyl; R2 is selected from a group consisting of aminoalkoxyl, alkyl/dialkyl aminoalkoxy, cyclic alkyl aminoalkoxy. R3 is selected from a group consisting of H, lower alkyl, lower alkoxy group such as methyl, ethyl, n-propyl, isopropyl, n-butyl, secondary butyl, tertiary butyl, n-amyl, n-hexyl, 2-ethyl butyl ; R4 is selected from a group consisting of H,OH,methyl etc.
(Formula Removed)
Background of the Invention
Tuberculosis is a growing international health concern; it is the leading infectious cause of death in the world today (Dolin, P.J. et al Bull. WHO 1994, 72, 213; Daffe, M. et al Adv. Microb. Physiol 1998, 39, 131). It is estimated that worldwide 100 million people are infected annually.
Approximately ten million develop the disease, with five million of these progressing to the infectious stage and ultimately three million dying. Even though improved methods of prevention, detection, diagnosis and modern treatment have greatly reduced the number of people getting infected and dying from it, the emergence of multi-drug-resistant (MDR) strains and the global human immunodeficiency virus (HIV) augments the risk of developing TB many fold. Resistance has been described for all first-line drugs (isoniazid, rifampin, pyrazinamide, ethambutol and streptomycin) and for several second-line and newer drugs (ethionamide, fluoroquinolones, macrolides, nitroimidazopyrans). Because MDR strains are the result of cumulative mutations, growth of Mycobacterium tuberculosis (MT) can successfully be controlled in the host by concomitant treatment with more than one drug. This has resulted in the development of new agents (Panda, G. et al Indian Journal of Chemistry, 2009, 48B, 1121-1127; Parai, M. K. et al Bioorganic & Medicinal Chemistry Letters, 2008, 18, 289-292) for the preparation of Disseminated Mycobacterium avium complex (DMAC) infection as well as combinations of both new and standard agents for its treatment. The search for more effective agents against Mycobacterium tuberculosis (MT) and Mycobacterium avium complex (MAC) is ongoing in an attempt to enhance survival and reduce morbidity, as proven by the high number of publications (Jing-Ping Lu et al J. Med. Chem., 2010, 53, 3, 1329-1337; Liqiang Chen et al J. Med. Chem., 2010, 53 (12), 4768-4778; Jiyoung A et al J. Med. Chem., 2009, 52 (17), 5485-5495; Maria-Teresa Gutierrez-Lugo et al J. Med. Chem., 2008, 51 (9), 2606-2612; Li Liu et al J. Med. Chem., 2010, 53 (7), 2882-2891 and references cited therein) and patents of new antituberculous drugs recently published. Preclinical data, such as in vitro measures of drug activity and pharmacokinetics, are used in the design of new treatment regimens. Assessment of pharmacodynamic activity from standard in vitro minimum inhibitory concentrations (MICs) alone is insufficient to predict in vivo potency. Achievable serum and tissue concentrations as well as pharmacokinetic characteristics must be considered.
Because of this, there is an urgent need for anti-TB drugs with improved properties such as enhanced activity against MDR strains, reduced toxicity, shortened duration of therapy, rapid mycobactericidal mechanism of action and the ability to penetrate host cells and exert anti-mycobacterial effects in the intracellular environment.
Following is the description of thiophene containing trisubstituted methanes having antimycobacterial activity.
PAPER
European Journal of Medicinal Chemistry (2015), 95, 357-368
Thiophene containing trisubstituted methanes [TRSMs] as identified lead against Mycobacterium tuberculosis
- a Medicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, B.S. 10/1, Jankipuram Extension, Sitapur Road, Lucknow-226031, UP, India
- b Biochemistry Division, CSIR-Central Drug Research Institute, B.S. 10/1, Jankipuram Extension, Sitapur Road, Lucknow-226031, UP, India
Triarylmethanes (TRAMs) and thiophene containing trisubstituted methanes (TRSMs) have been reported by us, having potential against Mycobacterium tuberculosis andMycobacterium fortuitum strains, respectively. Further, extension through synthesis and biological evaluation of novel TRSMs resulted into an identified lead 36 (S006-830) [(diisopropyl-(2-{4-[(4-methoxy-phenyl)- thiophen-2-yl-methyl]-phenoxy}-ethyl)-amine)] with MIC: 1.33 mg/L, non-toxic against Vero C-1008 cell line with selectivity index >10,ex vivo efficacy equivalent to first line TB drugs-isoniazid (INH), rifampicin (RFM) and pyrazinamide (PZA) in the mouse and human macrophages, and lung CFU count of 2.2 × 107 (approximately 15 fold lesser than untreated mice, 31 × 107) with efficacies comparable to ethambutol (EMB) (1.27 × 107) and PZA (1.9 × 107). Further, S006-830 also showed potent bactericidal activity against multi-drug resistant and single-drug resistant clinical isolates of M. tuberculosis
.
PAPER
Synthetic Communications (2014), 44(23), 3408-3413
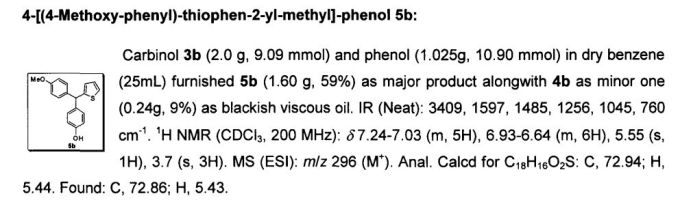
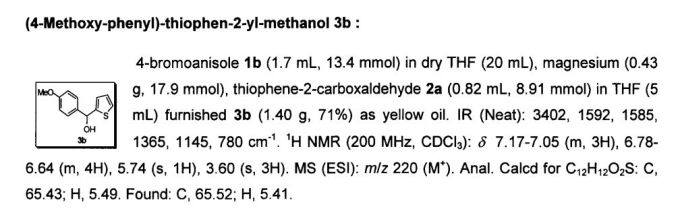
Abstract
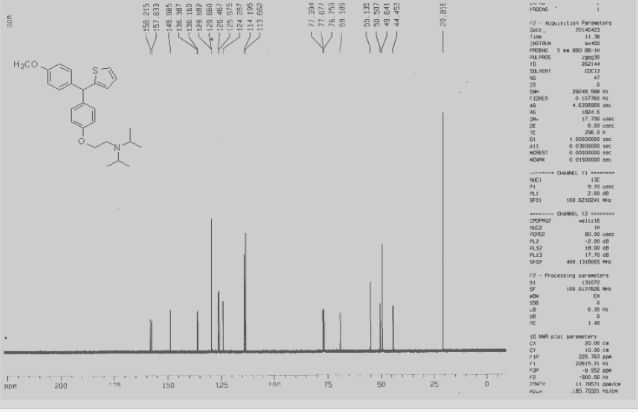
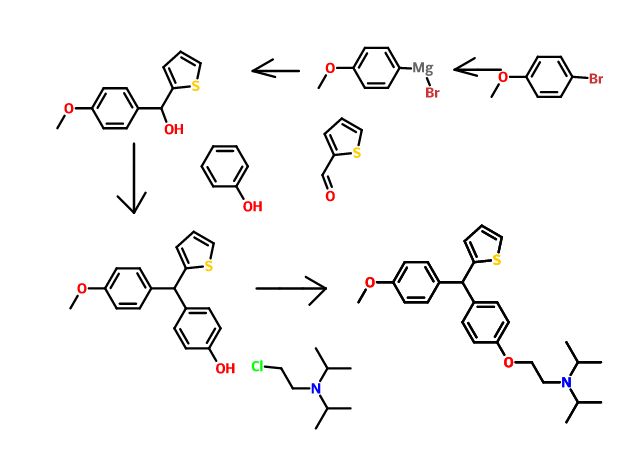
The triarylmethane antituberculosis drug CDRI-830 is synthesized. The triarylmethane derivative 4 is prepared from ether 6 by a rearrangement process. The total synthesis of the drug CDRI-830 is achieved in a good overall yield of 35% from a simple thiophene derivative 8.


Total Synthesis of an Experimental Antitubercular DrugDOI:
10.1080/00397911.2014.942745
pages 3408-3413
REFERENCES
S006-830 against H37RV, single, multi-drug resistant M. tuberculosis; CFU in the lungs with S006-830, EMB, PZA (European Journal of Medicinal Chemistry 2015, 95, 357-368, J Antimicrob Chemother. 2012; 67(5):1188-97, Bioorg Med Chem Lett, 2008, 18, 289-292)
Panda, G., Parai, M.K., Das, S.K., Shagufta, Sinha,M., Chaturvedi, V., Srivastava, A.K., Manju,
Y.S., Gaikwad, A.N., and Sinha, S.: Effect of substituents on diarylmethanes for antitubercular activity.
European Journal of Medicinal Chemistry;2007,42, 410-419
Drug Testing and Analysis (2016), 8(2), 180-188.
Current Pharmaceutical Analysis (2015), 11(1), 35-42.
Drug Testing and Analysis (2015), 7(8), 721-726
Indian Pat. Appl. (2012), IN 2010DE00685
| 1. DiaryloxyMethanoPhenanthrenes: A New Class of Antituberculosis Agents, G. Panda,Shagufta, Jitendra Kumar Mishra, Vinita Chaturvedi, Anil K. Srivastava, Manju, RanjanaSrivastava and Brahm S. Srivastava, 1178DEL2004 Filing date 24/06/04 | |
| 2. Thiophene containing Trisubstituted Methanes (TRSMs) as antitubercular agents, Gautam Panda, Maloy Kumar Parai, Priyanka Singh, Sudhir Sinha, Vinita Chaturvedi, Anil Gaikwad, PCT in process (685/DEL/2010) dt 20-2-2010 |
/////////S 006-830, CDRI 830, 1550975-42-2
c1c(ccc(c1)OC)C(c2ccc(cc2)OCCN(C(C)C)C(C)C)c3sccc3
/////////
Ajanta Caves, Aurangabad, maharashtra, india
 .
.











Ajanta Caves
Cave
The
Ajanta Caves in Aurangabad district of Maharashtra state of India are
about 30 rock-cut Buddhist cave monuments which date from the 2nd
century BCE to about 480 or 650 CE. Wikipedia
Address: Maharashtra 431117


/////////