Bis(4-methoxyphenyl)methanone
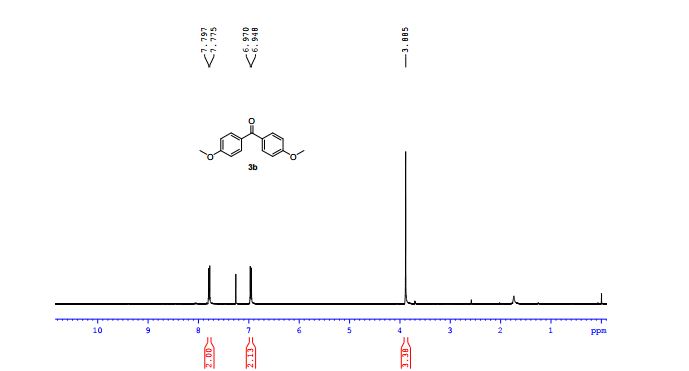
bis(4-methoxyphenyl)methanone (3b) The title product was purified by column chromatography and was obtained in 83% yield (110 mg). Rf = 0.3 (petroleum ether/ethyl acetate 30:1), light yellow oil.
1H NMR (400 MHz, CDCl3) δ (ppm):
7.80 (d, J = 8.8 Hz, 2H), AROM H ORTHO TO -C=0
6.97 (d, J = 8.8 Hz, 2H), AROM H ORTHO TO -OCH3
3.89 (s, 6H); TWO -OCH3 GPS
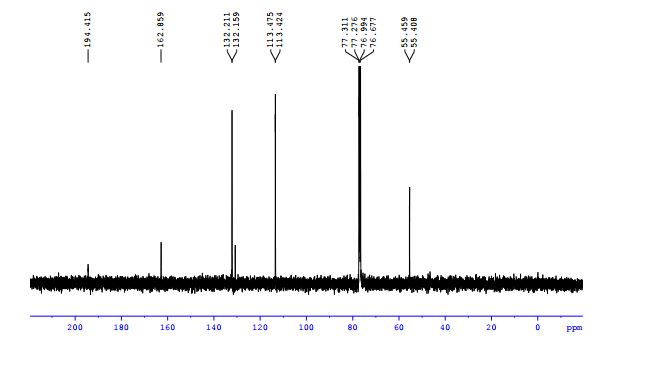
13C NMR (100 MHz, CDCl3) δ (ppm): 194.4, 162.9, 132.2, 132.1, 113.4, 55.5;
IR (KBr): 2957, 1671, 1593, 1260, 1093, 806 cm-1; HRMS(ESI) calc. for (M + Na+ ) 265.0844; found 265.0835.
MOM CAN TEACH YOU NMR
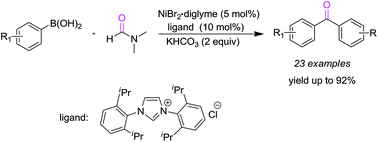
Nickel-catalyzed carbonylation of arylboronic acids with DMF as a CO source
Org. Chem. Front., 2017, 4,569-572
DOI: 10.1039/C7QO00001D, Research Article
DOI: 10.1039/C7QO00001D, Research Article
Yang Li, Dong-Huai Tu, Bo Wang, Ju-You Lu, Yao-Yu Wang, Zhao-Tie Liu, Zhong-Wen Liu, Jian Lu
By using N,N-dimethylformamide (DMF) as a CO source, nickel-catalyzed carbonylation of arylboronic acids was demonstrated as an efficient and facile protocol for the synthesis of diaryl ketones.
By using N,N-dimethylformamide (DMF) as a CO source, nickel-catalyzed carbonylation of arylboronic acids was demonstrated as an efficient and facile protocol for the synthesis of diaryl ketones.
Nickel-catalyzed carbonylation of arylboronic acids with DMF as a CO source
Abstract
By using N,N-dimethylformamide (DMF) as a CO source, the cheap metal nickel-catalyzed carbonylation of arylboronic acids was demonstrated as an efficient and facile protocol for the synthesis of diaryl ketones. Results indicated that NiBr2·diglyme was the best pre-catalyst among the investigated transitional metal salts, and excellent yields were achieved via C–H and C–N bond cleavage.