Green Chem., 2018, Advance Article
DOI: 10.1039/C7GC02862H, Paper
DOI: 10.1039/C7GC02862H, Paper
Peter Olsen, Michael Oschmann, Eric V. Johnston, Bjorn Akermark
Ring opening of cyclic carbonates with unprotected amino acids in water - a route to highly functional carbamates.
Ring opening of cyclic carbonates with unprotected amino acids in water - a route to highly functional carbamates.
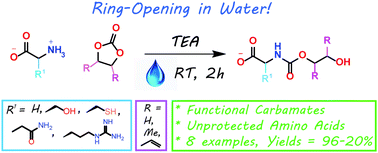
Synthesis of highly functional carbamates through ring-opening of cyclic carbonates with unprotected α-amino acids in water
Abstract
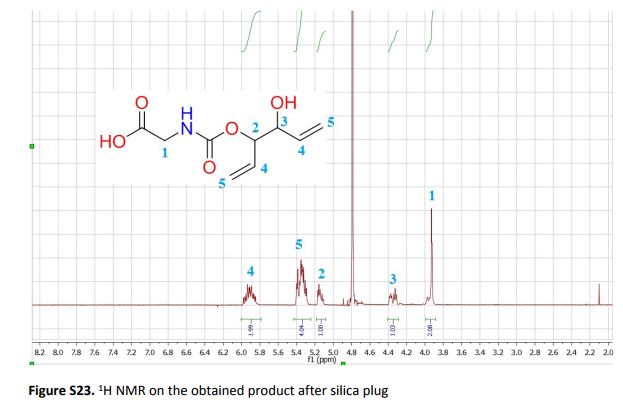
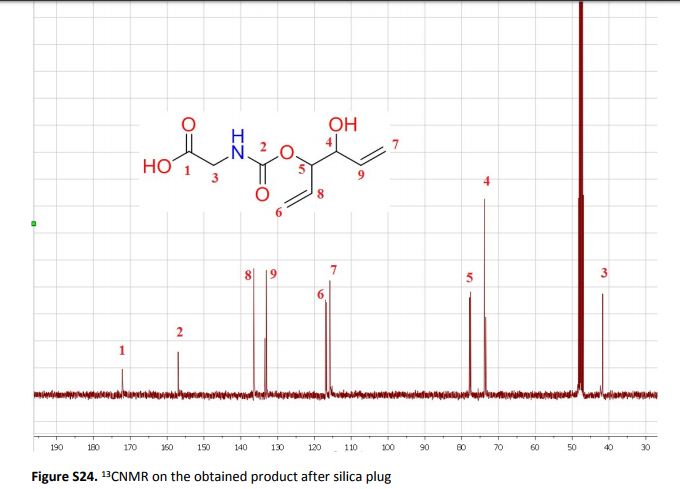
The present work shows that it is possible to ring-open cyclic carbonates with unprotected amino acids in water. Fine tuning of the reaction parameters made it possible to suppress the degree of hydrolysis in relation to aminolysis. This enabled the synthesis of functionally dense carbamates containing alkenes, carboxylic acids, alcohols and thiols after short reaction times at room temperature. When Glycine was used as the nucleophile in the ring-opening with four different five membered cyclic carbonates, containing a plethora of functional groups, the corresponding carbamates could be obtained in excellent yields (>90%) without the need for any further purification. Furthermore, the orthogonality of the transformation was explored through ring-opening of divinylenecarbonate with unprotected amino acids equipped with nucleophilic side chains, such as serine and cysteine. In these cases the reaction selectively produced the desired carbamate, in 70 and 50% yield respectively. The synthetic design provides an inexpensive and scalable protocol towards highly functionalized building blocks that are envisioned to find applications in both the small and macromolecular arena.
link http://pubs.rsc.org/en/Content/ArticleLanding/2018/GC/C7GC02862H?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
link http://pubs.rsc.org/en/Content/ArticleLanding/2018/GC/C7GC02862H?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract

Dr. Eric Johnston, Ph.D.

Chief Technology Officer
Dr. Eric V. Johnston obtained his Master of Science degree in 2008 at the Department of Organic Chemistry, Stockholm University, Sweden. In the same year, he started his graduate studies under the supervision of Prof. Jan-Erling Bäckvall. During his PhD, he worked on the development of new homogeneous and heterogeneous transition-metal catalysts.
After receiving his PhD in 2012, he joined Prof. Samuel J. Danishefskys research group at Memorial Sloan-Kettering Cancer Center, New York, USA as a postdoctoral fellow supported by The Swedish Research Council. Here he was engaged in the total chemical synthesis of glycolsylated proteins that play important roles in modern cancer treatment.
In 2014 he returned to the Department of Organic Chemistry at Stockholm University to establish his own group. The goal of his research is to contribute new advances to the strategy and methodology for the preparation of synthetic macromolecules such as proteins, glycopeptides, sequence and length-controlled polymers. He is also a Co-Supervisor for Prof. Björn Åkermarks research group, which aims at studying and developing new homogeneous, as well as heterogeneous, water oxidation catalysts.
//////////