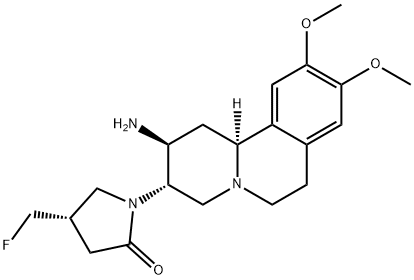
CARMEGLIPTIN
Org. Process Res. Dev. 2011, 15, 503–514. doi:10.1021/op2000207
http://pubs.acs.org/doi/full/10.1021/op2000207

A short and high-yielding synthesis of carmegliptin (1) suitable for large-scale production is reported. The tricyclic core was assembled efficiently by a decarboxylative Mannich addition−Mannich cyclization sequence. Subsequent crystallization-induced dynamic resolution of enamine 7 using (S,S)-dibenzoyltartaric acid was followed by diastereoselective enamine reduction to give the fully functionalized tricyclic core with its three stereogenic centers. The C-3 nitrogen was introduced by Hofmann rearrangement of amide 28, and the resulting amine 10was coupled with (S)-fluoromethyl lactone 31. Following cyclization to lactam 13 and amine deprotection, 1 was obtained in 27−31% overall yield with six isolated intermediates.
Preparation of (2S,3S,11βS)-1-(2-Amino-9,10-dimethoxy-1,3,4,6,7,11β-hexahydro-2H-pyrido[2,1-a]isoquinolin-3-yl)-(4S)-fluoromethyl-pyrrolidin-2-one Dihydrochloride (1) CARMEGLIPTIN
A suspension of carbamate 13 (136 kg, 285 mol) in a mixture of H2O (112 kg) and acetone (122 kg) was treated at 50 °C within 60 min with 37% aq HCl (98.0 kg). After 90 min at 47−52 °C the solution was polish filtered through a 5 μm filter. The first reactor and the transfer lines were washed with a hot (47−52 °C) mixture of H2O (13.0 kg) and acetone (116 kg). The filtrate was cooled to 25 °C and treated at this temperature within 80 min with acetone (1600 kg) whereupon the product crystallized out. The resulting suspension was stirred for 1 h at 25 °C and subsequently centrifuged. The crystals were washed in two portions with acetone (391 kg) and dried at 50 °C and <30 mbar until constant weight to afford 122.4 kg (95%) of the title compound as colorless crystals with an assay (HPLC) of 98.8% (w/w).
1H NMR (400 MHz, D2O) δ 2.11−2.22 (m, 1H); 2.45 (dd, J = 17.6 Hz, 6.7 Hz; 1H); 2.76 (dd, J = 17.6 Hz, 9.55 Hz, 1H); 2.90−3.05 (m, 1H); 3.08−3.19 (m, 2H); 3.24−3.36 (m, 1H); 3.43 (dd, J = 9.8 Hz, 5.75 Hz, 1H); 3.49−3.58 (m, 1H); 3.70−3.84 (m, 4H); 3.87 (s, 3H); 3.88 (s, 3H); 4.12 (td, J = 11.6 Hz, 4.5 Hz, 1H); 4.45−4.55 (m, 1H); 4.56−4.68 (m, 3H); 6.91 (s, 1H), 6.95 (s, 1H).
IR (cm−1): 3237, 2925, 1682, 496, 478.
MS (ESI): m/z 378.3 ([M + H]+ (free amine)).
Anal. Calcd for C20H30Cl2FN3O3: C, 53.34; H, 6.71; N, 9.33; Cl, 15.74; F 4.22; O, 10.66. Found: C, 53.04; H, 6.43; N, 9.45; Cl, 15.66; F, 4.29; O, 11.09.
REF FOR ABOVE
Mattei, P.; Böhringer, M.; Di Giorgio, P.; Fischer, H.; Hennig, M.; Huwyler, J.; Kocer, B.; Kuhn, B.; Löffler, B. M.; MacDonald, A.; Narquizian, R.; Rauber, E.; Sebokova, E.; Sprecher, U. Bioorg. Med. Chem. Lett. 2010, 20, 1109
Böhringer, M.; Kuhn, B.; Lübbers, T.; Mattei, P.; Narquizian, R.; Wessel,H. P. (F. Hoffmann-La Roche AG). U.S. Pat. Appl. 2004/0259902, 2004.